Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells
et al., Scientific Reports, doi:10.1038/s41598-022-19993-w, Sep 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
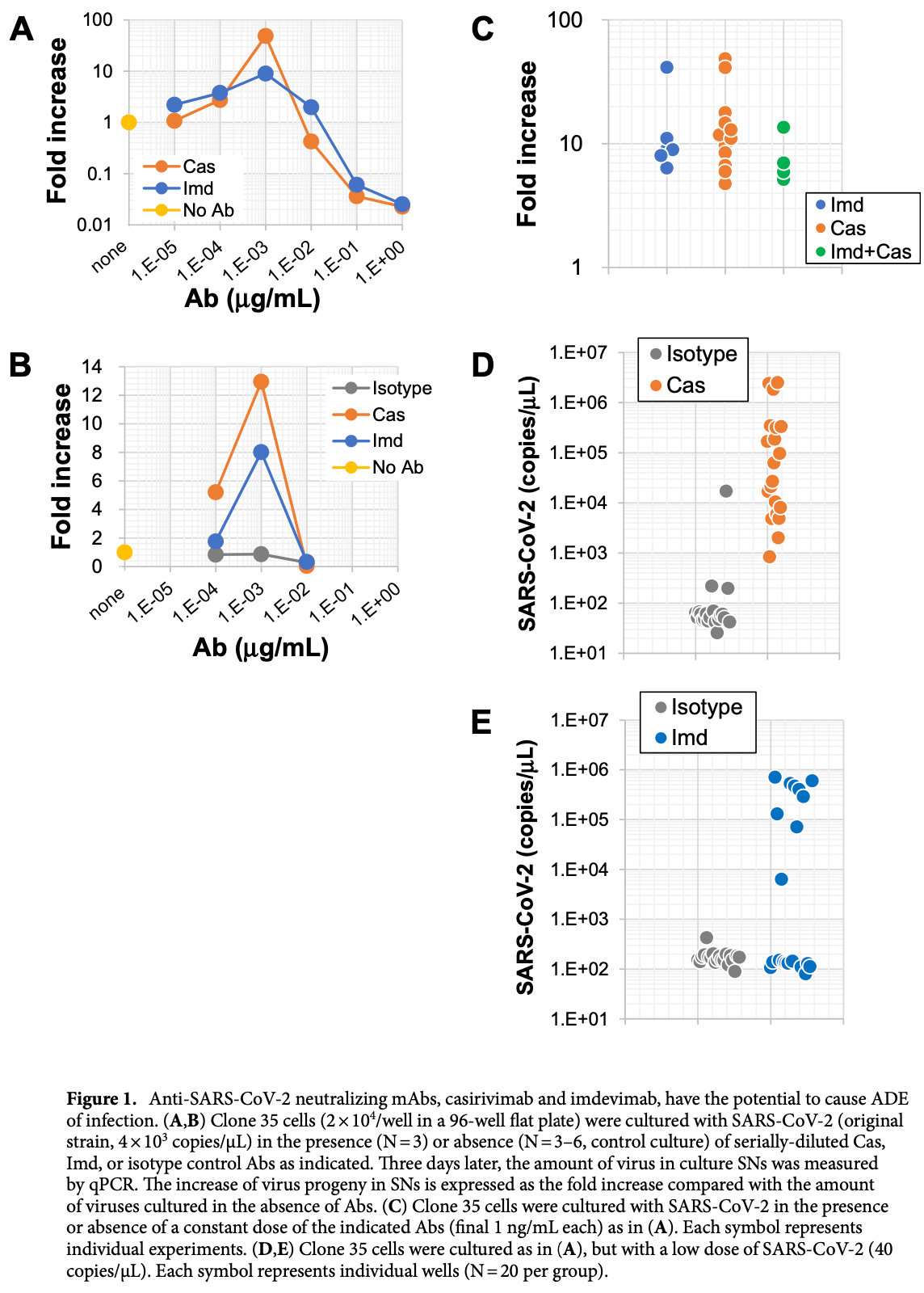
In vitro study showing that casirivimab/imdevimab may induce antibody-dependent enhancement (ADE) within a specific concentration range. No ADE was observed for sotrovimab.
Study covers sotrovimab and casirivimab/imdevimab.
Shimizu et al., 16 Sep 2022, Japan, peer-reviewed, 12 authors.
Contact: kmiyazaki@micantechnologies.com, shioda@biken.osaka-u.ac.jp.
Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells
Scientific Reports, doi:10.1038/s41598-022-19993-w
Many therapeutic antibodies (Abs) and mRNA vaccines, both targeting SARS-CoV-2 spike protein (S-protein), have been developed and approved in order to combat the ongoing COVID-19 pandemic. In consideration of these developments, a common concern has been the potential for Ab-dependent enhancement (ADE) of infection caused by inoculated or induced Abs. Although the preventive and therapeutic effects of these Abs are obvious, little attention has been paid to the influence of the remaining and dwindling anti-S-protein Abs in vivo. Here, we demonstrate that certain monoclonal Abs (mAbs) approved as therapeutic neutralizing anti-S-protein mAbs for human usage have the potential to cause ADE in a narrow range of Ab concentrations. Although sera collected from mRNAvaccinated individuals exhibited neutralizing activity, some sera gradually exhibited dominance of ADE activity in a time-dependent manner. None of the sera examined exhibited neutralizing activity against infection with the Omicron strain. Rather, some ADE of Omicron infection was observed in some sera. These results suggest the possible emergence of adverse effects caused by these Abs in addition to the therapeutic or preventive effect. Therapeutic Ab drugs targeting SARS-CoV-2 S-protein have shown high preventive efficacy against disease development 1-3 . In addition, current SARS-CoV-2 mRNA vaccines for humans also target the S-protein on viruses as a critical antigen 4 . These mRNA vaccines generate robust neutralizing Abs 5-7 , but for both Ab drugs and vaccines targeting the S-protein, the possible induction of Ab-dependent enhancement (ADE) of infection is a concern [8] [9] [10] [11] . Recent reports have demonstrated that neutralizing mAbs against S-protein can exhibit ADE activity in a limited window of Ab concentrations [12] [13] [14] . An important issue requiring reconsideration is that the cells used to evaluate ADE potential are different in each report. In many cases, Fc-receptor (FcR)-positive and angiotensin-converting enzyme 2 (ACE2, the major receptor for SARS-CoV-2 15-17 )-negative cells lines (Raji, THP-1, and K562) are used as host cells for infection of SARS-CoV-2 pseudo-viruses expressing S-protein or authentic SARS-CoV-2 [12] [13] [14] 18, 19 . These reports have demonstrated that some anti-S protein mAbs have the potential to induce ADE of infection. The observed ADE can be blocked in the presence of FcR-blocker, demonstrating FcR dependence. Likewise, the Ab drugs casirivimab and imdevimab 1, 20, 21 , which target the SARS-CoV-2 S-protein, have also been evaluated by using FcR-positive and ACE2-negative cell lines (U937, THP-1,
Methods Mylc cell lines for SARS-CoV-2 infection. The procedure used to generate Mylc lines was previously reported 38, 39 . Briefly, immortalized myeloid cell lines were established by the lentivirus-mediated transduction of cMYC, BMI-1, GM-CSF, and M-CSF into human iPS cell-derived myeloid cells. These cell lines were further induced to express ACE2 and TMPRSS2 using lentiviral vectors. The established cell lines (K-ML2(AT), D05, and PhF lines) were derived from different human iPS cells and maintained as bulk cell lines. In some experiments, cloned cells (clone 35) were established from bulk lines by limiting dilution. Each cell line was cultured in MEM alpha (Gibco) supplemented with 10% (vol/vol) fetal bovine serum under 37 °C, 5% CO 2 , and watersaturated humidity conditions. Viruses. SARS-CoV-2/Hu/DP/Kng/19-020 (original strain, GenBank accession number: LC528232.1) was provided by the Kanagawa Prefectural Institute of Public Health, Kanagawa, Japan, and the Omicron strain (hCoV-19/Japan/TY38-873/2021) by the National Institute of Infectious Diseases, Japan. SARS-CoV-2 Delta strain (hCoV-19/USA/PHC658/2021, NR-55611) was obtained from BEI Resources. SARS-CoV-2 viruses were passaged in VeroE6-TMPRSS2 cells (obtained from the National Institutes of Biomedical Innovation, Health, and Nutrition, JCRB cell bank, Japan) two times. Viral stocks were aliquoted, examined for the presence of viral RNA by quantitative RT-PCR, and tested for mycoplasma (they were..
References
Baden, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N. Engl. J. Med
Baum, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Castells, Phillips, Maintaining safety with SARS-CoV-2 vaccines, N. Engl. J. Med
Cathcart, The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2, bioRxiv
Cele, Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization, Nature
Copin, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell
Deeks, Casirivimab/Imdevimab: First approval, Drugs
Doria-Rose, Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19, N. Engl. J. Med
Gupta, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N. Engl. J. Med
Hadinegoro, Efficacy and long-term safety of a dengue vaccine in regions of endemic disease, N. Engl. J. Med
Hansen, Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail, Science
Haruta, Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1, Hum. Immunol
Haruta, TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigenpresenting cells, Gene Ther
Huisman, Martina, Rimmelzwaan, Gruters, Osterhaus, Vaccine-induced enhancement of viral infections, Vaccine
Kam, Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections, JCI Insight
Kim, A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat. Commun
Lee, Wheatley, Kent, Dekosky, Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies, Nat. Microbiol
Li, In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies, Cell
Liu, An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies, Cell
Logunov, Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia, The Lancet
Maemura, Antibody-dependent enhancement of SARS-CoV-2 infection is mediated By the IgG receptors FcgammaRIIA and FcgammaRIIIA but does not contribute to aberrant cytokine production by macrophages, mBio
Mannar, Leopold, Subramaniam, Glycan reactive anti-HIV-1 antibodies bind the SARS-CoV-2 spike protein but do not block viral entry, Sci. Rep
Meng, Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts tropism and fusogenicity, Nature
Monteil, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell
Qin, Genome characterization and potential risk assessment of the novel SARS-CoV-2 variant omicron, B, doi:10.15212/ZOONOSES-2021-0024
Sadoff, Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19, N. Engl. J. Med
Scialo, ACE2: The major cell entry receptor for SARS-CoV-2, Lung
Shimizu, The potential of COVID-19 patients' sera to cause antibody-dependent enhancement of infection and IL-6 production, Sci. Rep
Takashita, Efficacy of antibodies and antiviral drugs against covid-19 omicron variant, N. Engl. J. Med
Viana, Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa, Nature
Walls, Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell
Wang, ACE2 can act as the secondary receptor in the FcγR-dependent ADE of SARS-CoV-2 infection, iScience
Wang, Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys, Nat. Commun
Weinreich, REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19, N. Engl. J. Med
Xu, A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus, NPJ Vaccines
Zhou, Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD, Cell Rep
Zou, Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection, Nat. Commun
DOI record:
{
"DOI": "10.1038/s41598-022-19993-w",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-022-19993-w",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Many therapeutic antibodies (Abs) and mRNA vaccines, both targeting SARS-CoV-2 spike protein (S-protein), have been developed and approved in order to combat the ongoing COVID-19 pandemic. In consideration of these developments, a common concern has been the potential for Ab-dependent enhancement (ADE) of infection caused by inoculated or induced Abs. Although the preventive and therapeutic effects of these Abs are obvious, little attention has been paid to the influence of the remaining and dwindling anti-S-protein Abs in vivo. Here, we demonstrate that certain monoclonal Abs (mAbs) approved as therapeutic neutralizing anti-S-protein mAbs for human usage have the potential to cause ADE in a narrow range of Ab concentrations. Although sera collected from mRNA-vaccinated individuals exhibited neutralizing activity, some sera gradually exhibited dominance of ADE activity in a time-dependent manner. None of the sera examined exhibited neutralizing activity against infection with the Omicron strain. Rather, some ADE of Omicron infection was observed in some sera. These results suggest the possible emergence of adverse effects caused by these Abs in addition to the therapeutic or preventive effect.</jats:p>",
"alternative-id": [
"19993"
],
"article-number": "15612",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 May 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "7 September 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "16 September 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Shimizu",
"given": "Jun",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sasaki",
"given": "Tadahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koketsu",
"given": "Ritsuko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morita",
"given": "Ryo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoshimura",
"given": "Yuka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murakami",
"given": "Ami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saito",
"given": "Yua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kusunoki",
"given": "Toshie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samune",
"given": "Yoshihiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nakayama",
"given": "Emi E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Miyazaki",
"given": "Kazuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shioda",
"given": "Tatsuo",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
16
]
],
"date-time": "2022-09-16T16:04:09Z",
"timestamp": 1663344249000
},
"deposited": {
"date-parts": [
[
2022,
9,
16
]
],
"date-time": "2022-09-16T16:17:13Z",
"timestamp": 1663345033000
},
"funder": [
{
"DOI": "10.13039/100009619",
"award": [
"JP20he0822004"
],
"doi-asserted-by": "crossref",
"name": "the Japan Agency for Medical Research and Development"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T12:53:44Z",
"timestamp": 1672145624652
},
"is-referenced-by-count": 2,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
16
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
16
]
],
"date-time": "2022-09-16T00:00:00Z",
"timestamp": 1663286400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
16
]
],
"date-time": "2022-09-16T00:00:00Z",
"timestamp": 1663286400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-022-19993-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-19993-w",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-022-19993-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
9,
16
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
16
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1126/science.abd0827",
"author": "J Hansen",
"doi-asserted-by": "publisher",
"first-page": "1010",
"journal-title": "Science",
"key": "19993_CR1",
"unstructured": "Hansen, J. et al. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science 369, 1010–1014 (2020).",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR2",
"unstructured": "Weinreich, D. M. et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 384, 238–251 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1007/s40265-021-01620-z",
"author": "ED Deeks",
"doi-asserted-by": "publisher",
"first-page": "2047",
"journal-title": "Drugs",
"key": "19993_CR3",
"unstructured": "Deeks, E. D. Casirivimab/Imdevimab: First approval. Drugs 81, 2047–2055 (2021).",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1056/NEJMra2035343",
"author": "MC Castells",
"doi-asserted-by": "publisher",
"first-page": "643",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR4",
"unstructured": "Castells, M. C. & Phillips, E. J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 384, 643–649 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2101544",
"author": "J Sadoff",
"doi-asserted-by": "publisher",
"first-page": "2187",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR5",
"unstructured": "Sadoff, J. et al. Safety and efficacy of single-dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 384, 2187–2201 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035389",
"author": "LR Baden",
"doi-asserted-by": "publisher",
"first-page": "403",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR6",
"unstructured": "Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00234-8",
"author": "DY Logunov",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "The Lancet",
"key": "19993_CR7",
"unstructured": "Logunov, D. Y. et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. The Lancet 397, 671–681 (2021).",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1038/s41541-016-0003-3",
"author": "M Xu",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "NPJ Vaccines",
"key": "19993_CR8",
"unstructured": "Xu, M. et al. A potent neutralizing antibody with therapeutic potential against all four serotypes of dengue virus. NPJ Vaccines 2, 2 (2017).",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1016/j.vaccine.2008.10.087",
"author": "W Huisman",
"doi-asserted-by": "publisher",
"first-page": "505",
"journal-title": "Vaccine",
"key": "19993_CR9",
"unstructured": "Huisman, W., Martina, B. E., Rimmelzwaan, G. F., Gruters, R. A. & Osterhaus, A. D. Vaccine-induced enhancement of viral infections. Vaccine 27, 505–512 (2009).",
"volume": "27",
"year": "2009"
},
{
"DOI": "10.1056/NEJMoa1506223",
"author": "SR Hadinegoro",
"doi-asserted-by": "publisher",
"first-page": "1195",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR10",
"unstructured": "Hadinegoro, S. R. et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Engl. J. Med. 373, 1195–1206 (2015).",
"volume": "373",
"year": "2015"
},
{
"DOI": "10.1038/s41564-020-00789-5",
"author": "WS Lee",
"doi-asserted-by": "publisher",
"first-page": "1185",
"journal-title": "Nat. Microbiol.",
"key": "19993_CR11",
"unstructured": "Lee, W. S., Wheatley, A. K., Kent, S. J. & DeKosky, B. J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 5, 1185–1191 (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.06.021",
"author": "D Li",
"doi-asserted-by": "publisher",
"first-page": "4203",
"journal-title": "Cell",
"key": "19993_CR12",
"unstructured": "Li, D. et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell 184, 4203–4219 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-19568-1",
"author": "S Wang",
"doi-asserted-by": "publisher",
"first-page": "5752",
"journal-title": "Nat. Commun.",
"key": "19993_CR13",
"unstructured": "Wang, S. et al. Characterization of neutralizing antibody with prophylactic and therapeutic efficacy against SARS-CoV-2 in rhesus monkeys. Nat. Commun. 11, 5752 (2020).",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.celrep.2021.108699",
"author": "Y Zhou",
"doi-asserted-by": "publisher",
"journal-title": "Cell Rep.",
"key": "19993_CR14",
"unstructured": "Zhou, Y. et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associates with distinct epitopes on the RBD. Cell Rep. 34, 108699 (2021).",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1007/s00408-020-00408-4",
"author": "F Scialo",
"doi-asserted-by": "publisher",
"first-page": "867",
"journal-title": "Lung",
"key": "19993_CR15",
"unstructured": "Scialo, F. et al. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 198, 867–877 (2020).",
"volume": "198",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"author": "AC Walls",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Cell",
"key": "19993_CR16",
"unstructured": "Walls, A. C. et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"author": "V Monteil",
"doi-asserted-by": "publisher",
"first-page": "905",
"journal-title": "Cell",
"key": "19993_CR17",
"unstructured": "Monteil, V. et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181, 905–913 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"author": "C Kim",
"doi-asserted-by": "publisher",
"first-page": "288",
"journal-title": "Nat. Commun.",
"key": "19993_CR18",
"unstructured": "Kim, C. et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 12, 288 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-91746-7",
"author": "D Mannar",
"doi-asserted-by": "publisher",
"first-page": "12448",
"journal-title": "Sci. Rep.",
"key": "19993_CR19",
"unstructured": "Mannar, D., Leopold, K. & Subramaniam, S. Glycan reactive anti-HIV-1 antibodies bind the SARS-CoV-2 spike protein but do not block viral entry. Sci. Rep. 11, 12448 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.06.002",
"author": "R Copin",
"doi-asserted-by": "publisher",
"first-page": "3949",
"journal-title": "Cell",
"key": "19993_CR20",
"unstructured": "Copin, R. et al. The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies. Cell 184, 3949–3961 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1126/science.abd0831",
"author": "A Baum",
"doi-asserted-by": "publisher",
"first-page": "1014",
"journal-title": "Science",
"key": "19993_CR21",
"unstructured": "Baum, A. et al. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science 369, 1014–1018 (2020).",
"volume": "369",
"year": "2020"
},
{
"key": "19993_CR22",
"unstructured": "Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Regen-Cov (Casirivimab and Imdevimab) https://www.fda.gov/media/145611/download.) (2021)."
},
{
"DOI": "10.1128/mBio.01987-21",
"author": "T Maemura",
"doi-asserted-by": "publisher",
"first-page": "e0198721",
"journal-title": "mBio",
"key": "19993_CR23",
"unstructured": "Maemura, T. et al. Antibody-dependent enhancement of SARS-CoV-2 infection is mediated By the IgG receptors FcgammaRIIA and FcgammaRIIIA but does not contribute to aberrant cytokine production by macrophages. mBio 12, e0198721 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2021.103720",
"author": "Z Wang",
"doi-asserted-by": "publisher",
"first-page": "103720",
"journal-title": "iScience",
"key": "19993_CR24",
"unstructured": "Wang, Z. et al. ACE2 can act as the secondary receptor in the FcγR-dependent ADE of SARS-CoV-2 infection. iScience 25, 103720 (2022).",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-03273-0",
"author": "J Shimizu",
"doi-asserted-by": "publisher",
"first-page": "23713",
"journal-title": "Sci. Rep.",
"key": "19993_CR25",
"unstructured": "Shimizu, J. et al. The potential of COVID-19 patients’ sera to cause antibody-dependent enhancement of infection and IL-6 production. Sci. Rep. 11, 23713 (2021).",
"volume": "11",
"year": "2021"
},
{
"author": "AL Cathcart",
"first-page": "1123",
"journal-title": "bioRxiv",
"key": "19993_CR26",
"unstructured": "Cathcart, A. L. et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv 35, 1123 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1941",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR27",
"unstructured": "Gupta, A. et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N. Engl. J. Med. 385, 1941–1950 (2021).",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04411-y",
"author": "R Viana",
"doi-asserted-by": "publisher",
"first-page": "679",
"journal-title": "Nature",
"key": "19993_CR28",
"unstructured": "Viana, R. et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 603, 679–686 (2022).",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"author": "E Takashita",
"doi-asserted-by": "publisher",
"first-page": "995",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR29",
"unstructured": "Takashita, E. et al. Efficacy of antibodies and antiviral drugs against covid-19 omicron variant. N. Engl. J. Med. 386, 995–998 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2021.05.032",
"author": "Y Liu",
"doi-asserted-by": "publisher",
"first-page": "3452",
"journal-title": "Cell",
"key": "19993_CR30",
"unstructured": "Liu, Y. et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell 184, 3452–3466 (2021).",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2103916",
"author": "N Doria-Rose",
"doi-asserted-by": "publisher",
"first-page": "2259",
"journal-title": "N. Engl. J. Med.",
"key": "19993_CR31",
"unstructured": "Doria-Rose, N. et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N. Engl. J. Med. 384, 2259–2261 (2021).",
"volume": "384",
"year": "2021"
},
{
"key": "19993_CR32",
"unstructured": "Science Brief: SARS-CoV-2 Infection-induced and Vaccine-induced Immunity (https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine-induced-immunity.html).) (2021)."
},
{
"DOI": "10.15212/ZOONOSES-2021-0024",
"author": "S Qin",
"doi-asserted-by": "publisher",
"journal-title": "Zoonoses",
"key": "19993_CR33",
"unstructured": "Qin, S. et al. Genome characterization and potential risk assessment of the novel SARS-CoV-2 variant omicron (B.1.1.529). Zoonoses https://doi.org/10.15212/ZOONOSES-2021-0024 (2021).",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04387-1",
"author": "S Cele",
"doi-asserted-by": "publisher",
"first-page": "654",
"journal-title": "Nature",
"key": "19993_CR34",
"unstructured": "Cele, S. et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656 (2021).",
"volume": "602",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"author": "B Meng",
"doi-asserted-by": "publisher",
"first-page": "706",
"journal-title": "Nature",
"key": "19993_CR35",
"unstructured": "Meng, B. et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts tropism and fusogenicity. Nature 603, 706–714 (2022).",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-28544-w",
"author": "J Zou",
"doi-asserted-by": "publisher",
"first-page": "852",
"journal-title": "Nat. Commun.",
"key": "19993_CR36",
"unstructured": "Zou, J. et al. Neutralization against Omicron SARS-CoV-2 from previous non-Omicron infection. Nat. Commun. 13, 852 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1172/jci.insight.92428",
"author": "YW Kam",
"doi-asserted-by": "publisher",
"journal-title": "JCI Insight",
"key": "19993_CR37",
"unstructured": "Kam, Y. W. et al. Cross-reactive dengue human monoclonal antibody prevents severe pathologies and death from Zika virus infections. JCI Insight 2, e92428 (2017).",
"volume": "2",
"year": "2017"
},
{
"DOI": "10.1016/j.humimm.2013.05.017",
"author": "M Haruta",
"doi-asserted-by": "publisher",
"first-page": "1400",
"journal-title": "Hum. Immunol.",
"key": "19993_CR38",
"unstructured": "Haruta, M. et al. Generation of a large number of functional dendritic cells from human monocytes expanded by forced expression of cMYC plus BMI1. Hum. Immunol. 74, 1400–1408 (2013).",
"volume": "74",
"year": "2013"
},
{
"DOI": "10.1038/gt.2012.59",
"author": "M Haruta",
"doi-asserted-by": "publisher",
"first-page": "504",
"journal-title": "Gene Ther.",
"key": "19993_CR39",
"unstructured": "Haruta, M. et al. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 20, 504–513 (2013).",
"volume": "20",
"year": "2013"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-022-19993-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Reevaluation of antibody-dependent enhancement of infection in anti-SARS-CoV-2 therapeutic antibodies and mRNA-vaccine antisera using FcR- and ACE2-positive cells",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "12"
}
