Different Modalities in the Management of Post-COVID-19 Olfactory Dysfunction
et al., Indian Journal of Otolaryngology and Head & Neck Surgery, doi:10.1007/s12070-024-05213-6, Nov 2024
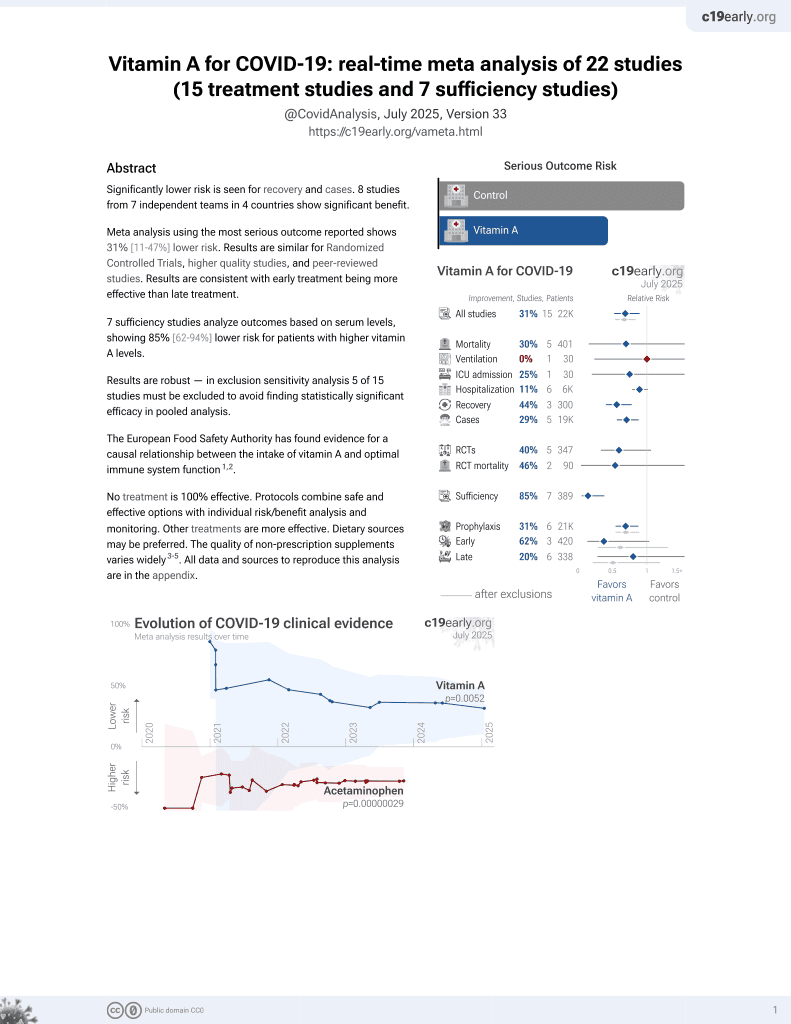
Vitamin A for COVID-19
49th treatment shown to reduce risk in
May 2023, now with p = 0.004 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
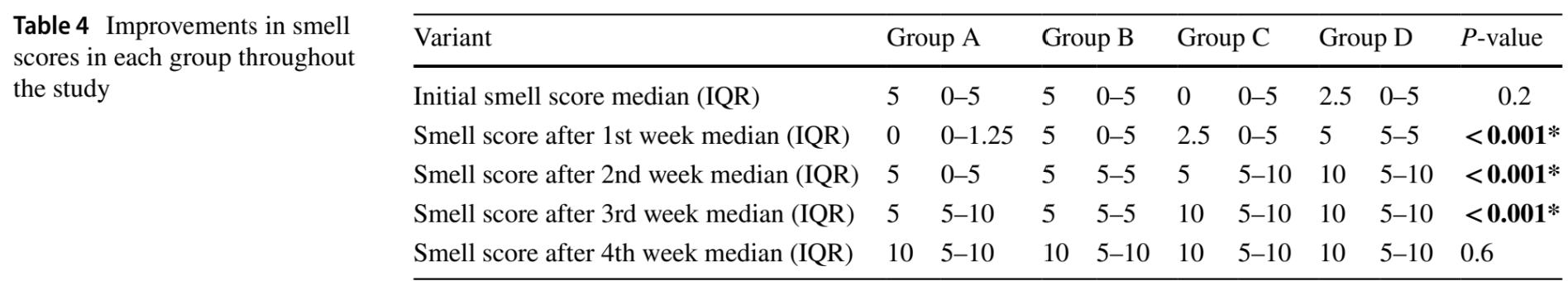
Retrospective 120 cases showing that topical mometasone furoate, vitamin A, or intranasal theophylline shortened time to recovery from post-COVID-19 olfactory dysfunction compared to olfactory training alone. Final smell scores after four weeks were not significantly different between groups. Comorbidities significantly impacted recovery time.
Saleh et al., 19 Nov 2024, retrospective, Saudi Arabia, peer-reviewed, 4 authors.
DOI record:
{
"DOI": "10.1007/s12070-024-05213-6",
"ISSN": [
"2231-3796",
"0973-7707"
],
"URL": "http://dx.doi.org/10.1007/s12070-024-05213-6",
"alternative-id": [
"5213"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "26 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "6 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "19 November 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no conflict of interest."
},
{
"group": {
"label": "Ethical and consent",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "This study was approved by the Benha Faculty of Medicine’s ethical committee (REC-FOMBU), Egypt, which approved the study protocol with the approval number RC-17–1-2023 and approval of the scientific committee at Dallah hospital to carry out this study. The study was carried out in compliance with the Helsinki Declaration of 1975 and its amendments. A written informed consent form was obtained."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "Not applicable."
},
{
"group": {
"label": "Permission to reproduce material from other sources",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 5,
"value": "The corresponding author is responsible for obtaining written permission to reproduce the material \"in print and other media\" from the publisher of the original source, and for supplying Springer with that permission. A rightslink facility for requesting permission from Wiley journals is available on each journal's website. The corresponding author is also responsible for completing and returning to the editorial office or the publisher the journal-specific copyright transfer form, and any financial disclosure forms that might be required for a particular journal. These forms are usually found on each journal's website. Forms may also be available from the journal's editorial office."
},
{
"group": {
"label": "Patient consent and Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 6,
"value": "This study was approved by the Benha Faculty of Medicine’s ethical committee (REC-FOMBU), Egypt, which approved the study protocol with the approval number RC-17–1-2023 and approval of the scientific committee at Dallah hospital to carry out this study. The study was carried out in compliance with the Helsinki Declaration of 1975 and its amendments. A written informed consent form was obtained from all patients who participated in this study. All authors of this research confirm their consent for publcation of this study."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8584-5106",
"affiliation": [],
"authenticated-orcid": false,
"family": "Saleh",
"given": "Ahmed Shehata El Sayed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mohamady",
"given": "Ayman Abdelaal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sobhey",
"given": "Mostafa Gomaa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9161-3149",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shady",
"given": "Eslam Farid Abu",
"sequence": "additional"
}
],
"container-title": "Indian Journal of Otolaryngology and Head & Neck Surgery",
"container-title-short": "Indian J Otolaryngol Head Neck Surg",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T16:13:51Z",
"timestamp": 1732032831000
},
"deposited": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T16:51:31Z",
"timestamp": 1732035091000
},
"indexed": {
"date-parts": [
[
2024,
11,
20
]
],
"date-time": "2024-11-20T05:33:54Z",
"timestamp": 1732080834938,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
11,
19
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T00:00:00Z",
"timestamp": 1731974400000
}
},
{
"URL": "https://www.springernature.com/gp/researchers/text-and-data-mining",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T00:00:00Z",
"timestamp": 1731974400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12070-024-05213-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s12070-024-05213-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s12070-024-05213-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1007",
"published": {
"date-parts": [
[
2024,
11,
19
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
19
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1080/14787210.2020.1792289",
"author": "G Cherry",
"doi-asserted-by": "publisher",
"first-page": "1165",
"issue": "11",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "5213_CR1",
"unstructured": "Cherry G, Rocke J, Chu M, Liu J, Lechner M, Lund VJ et al (2020) Loss of smell and taste: a new marker of COVID-19? Tracking reduced sense of smell during the coronavirus pandemic using search trends. Expert Rev Anti Infect Ther 18(11):1165–1170",
"volume": "18",
"year": "2020"
},
{
"author": "J Kanjanaumporn",
"first-page": "69",
"issue": "2",
"journal-title": "Asian Pac J Allergy Immunol",
"key": "5213_CR2",
"unstructured": "Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S (2020) Smell and taste dysfunction in patients with SARS-CoV-2 infection: a review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol 38(2):69–77",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1093/chemse/bjx025",
"author": "S Boesveldt",
"doi-asserted-by": "publisher",
"first-page": "513",
"issue": "7",
"journal-title": "Chem Senses",
"key": "5213_CR3",
"unstructured": "Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD et al (2017) Anosmia—a clinical review. Chem Senses 42(7):513–523",
"volume": "42",
"year": "2017"
},
{
"DOI": "10.2500/ajra.2014.28.4102",
"author": "DY Lee",
"doi-asserted-by": "publisher",
"first-page": "419",
"issue": "5",
"journal-title": "Am J Rhinol Allergy",
"key": "5213_CR4",
"unstructured": "Lee DY, Lee WH, Wee JH, Kim J-W (2014) Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy 28(5):419–422",
"volume": "28",
"year": "2014"
},
{
"DOI": "10.1007/s40629-022-00216-7",
"author": "L Klimek",
"doi-asserted-by": "publisher",
"first-page": "243",
"issue": "7",
"journal-title": "Allergo journal international",
"key": "5213_CR5",
"unstructured": "Klimek L, Hagemann J, Döge J, Freudelsperger L, Cuevas M, Klimek F et al (2022) Olfactory and gustatory disorders in COVID-19. Allergo journal international 31(7):243–250",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.1007/s00011-021-01446-1",
"author": "J Li",
"doi-asserted-by": "publisher",
"first-page": "407",
"journal-title": "Inflamm Res",
"key": "5213_CR6",
"unstructured": "Li J, Liu H-H, Yin X-D, Li C-C, Wang J (2021) COVID-19 illness and autoimmune diseases: recent insights. Inflamm Res 70:407–428",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.2174/1570159X20666220420113513",
"author": "A Di Stadio",
"doi-asserted-by": "publisher",
"first-page": "2001",
"issue": "10",
"journal-title": "Curr Neuropharmacol",
"key": "5213_CR7",
"unstructured": "Di Stadio A, D’Ascanio L, Vaira LA, Cantone E, De Luca P, Cingolani C et al (2022) Ultramicronized palmitoylethanolamide and luteolin supplement combined with olfactory training to treat post-COVID-19 olfactory impairment: a multi-center double-blinded randomized placebo-controlled clinical trial. Curr Neuropharmacol 20(10):2001–2012",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.3390/pathogens10060698",
"author": "S Saussez",
"doi-asserted-by": "publisher",
"first-page": "698",
"issue": "06",
"journal-title": "Pathogens",
"key": "5213_CR8",
"unstructured": "Saussez S, Vaira LA, Chiesa-Estomba CM, Le Bon SD, Horoi M, Deiana G et al (2021) Short-term efficacy and safety of oral and nasal corticosteroids in COVID-19 patients with olfactory dysfunction: a European multicenter study. Pathogens 10(06):698",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1001/archotol.1962.00740040122008",
"author": "RB Duncan",
"doi-asserted-by": "publisher",
"first-page": "116",
"issue": "2",
"journal-title": "Arch Otolaryngol",
"key": "5213_CR9",
"unstructured": "Duncan RB, Briggs M (1962) Treatment of uncomplicated anosmia by vitamin A. Arch Otolaryngol 75(2):116–124",
"volume": "75",
"year": "1962"
},
{
"DOI": "10.1007/s00405-017-4576-x",
"author": "T Hummel",
"doi-asserted-by": "publisher",
"first-page": "2819",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "5213_CR10",
"unstructured": "Hummel T, Whitcroft KL, Rueter G, Haehner A (2017) Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur Arch Otorhinolaryngol 274:2819–2825",
"volume": "274",
"year": "2017"
},
{
"DOI": "10.1097/MAJ.0b013e3181914a97",
"author": "RI Henkin",
"doi-asserted-by": "publisher",
"first-page": "396",
"issue": "6",
"journal-title": "Am J Med Sci",
"key": "5213_CR11",
"unstructured": "Henkin RI, Velicu I, Schmidt L (2009) An open-label controlled trial of theophylline for treatment of patients with hyposmia. Am J Med Sci 337(6):396–406",
"volume": "337",
"year": "2009"
},
{
"DOI": "10.1001/2013.jamaoto.342",
"author": "RI Henkin",
"doi-asserted-by": "publisher",
"first-page": "1064",
"issue": "11",
"journal-title": "Arch Otolaryngol-Head Neck Surg",
"key": "5213_CR12",
"unstructured": "Henkin RI, Schultz M, Minnick-Poppe L (2012) Intranasal theophylline treatment of hyposmia and hypogeusia: a pilot study. Arch Otolaryngol-Head Neck Surg 138(11):1064–1070",
"volume": "138",
"year": "2012"
},
{
"DOI": "10.1016/j.annemergmed.2020.07.022",
"author": "AD Haimovich",
"doi-asserted-by": "publisher",
"first-page": "442",
"issue": "4",
"journal-title": "Ann Emerg Med",
"key": "5213_CR13",
"unstructured": "Haimovich AD, Ravindra NG, Stoytchev S, Young HP, Wilson FP, Van Dijk D et al (2020) Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med 76(4):442–453",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/j.anl.2020.06.003",
"author": "MW El-Anwar",
"doi-asserted-by": "publisher",
"first-page": "559",
"issue": "4",
"journal-title": "Auris Nasus Larynx",
"key": "5213_CR14",
"unstructured": "El-Anwar MW, Elzayat S, Fouad YA (2020) ENT manifestation in COVID-19 patients. Auris Nasus Larynx 47(4):559–564",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa525",
"author": "J Luers",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "5213_CR15",
"unstructured": "Luers J, Rokohl A, Loreck N, Wawer Matos P, Augustin M, Dewald F (2020) Olfactory and gustatory dysfunction in coronavirus disease 19 (COVID-19). Clin Infect Dis. https://doi.org/10.1093/cid/ciaa525",
"year": "2020"
},
{
"DOI": "10.1007/s00405-020-06520-8",
"author": "S-D Le Bon",
"doi-asserted-by": "publisher",
"first-page": "3113",
"journal-title": "Eur Arch Otorhinolaryngol",
"key": "5213_CR16",
"unstructured": "Le Bon S-D, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M (2021) Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID-19-related loss of smell. Eur Arch Otorhinolaryngol 278:3113–3117",
"volume": "278",
"year": "2021"
},
{
"DOI": "10.1016/j.amjoto.2020.102884",
"author": "AA Abdelalim",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Am J Otolaryngol",
"key": "5213_CR17",
"unstructured": "Abdelalim AA, Mohamady AA, Elsayed RA, Elawady MA, Ghallab AF (2021) Corticosteroid nasal spray for recovery of smell sensation in COVID-19 patients: a randomized controlled trial. Am J Otolaryngol 42(2):102884",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.3390/life12101483",
"author": "CA Hintschich",
"doi-asserted-by": "publisher",
"first-page": "1483",
"issue": "10",
"journal-title": "Life",
"key": "5213_CR18",
"unstructured": "Hintschich CA, Dietz M, Haehner A, Hummel T (2022) Topical administration of mometasone is not helpful in post-COVID-19 olfactory dysfunction. Life 12(10):1483",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1001/jamaoto.2022.1573",
"author": "S Gupta",
"doi-asserted-by": "publisher",
"first-page": "830",
"issue": "9",
"journal-title": "JAMA Otolaryngol-Head Neck Surg",
"key": "5213_CR19",
"unstructured": "Gupta S, Lee JJ, Perrin A, Khan A, Smith HJ, Farrell N et al (2022) Efficacy and safety of saline nasal irrigation plus theophylline for treatment of COVID-19–related olfactory dysfunction: the SCENT2 phase 2 randomized clinical trial. JAMA Otolaryngol-Head Neck Surg 148(9):830–837",
"volume": "148",
"year": "2022"
},
{
"DOI": "10.1016/j.amjoto.2021.103299",
"author": "JJ Lee",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Am J Otolaryngol",
"key": "5213_CR20",
"unstructured": "Lee JJ, Peterson AM, Kallogjeri D, Jiramongkolchai P, Kukuljan S, Schneider JS et al (2022) Smell changes and efficacy of nasal theophylline (SCENT) irrigation: a randomized controlled trial for treatment of post-viral olfactory dysfunction. Am J Otolaryngol 43(2):103299",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1177/0194599820934761",
"author": "A Lovato",
"doi-asserted-by": "publisher",
"first-page": "852",
"issue": "4",
"journal-title": "Otolaryngol-Head Neck Surg",
"key": "5213_CR21",
"unstructured": "Lovato A, Antonini A, de Filippis C (2020) Comment on “The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis.” Otolaryngol-Head Neck Surg 163(4):852",
"volume": "163",
"year": "2020"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s12070-024-05213-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Different Modalities in the Management of Post-COVID-19 Olfactory Dysfunction",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}