Effect of lactoferrin treatment on symptoms and physical performance in long COVID patients: a randomised, double-blind, placebo-controlled trial
et al., ERJ Open Research, doi:10.1183/23120541.00031-2024, LARGO, Mar 2024
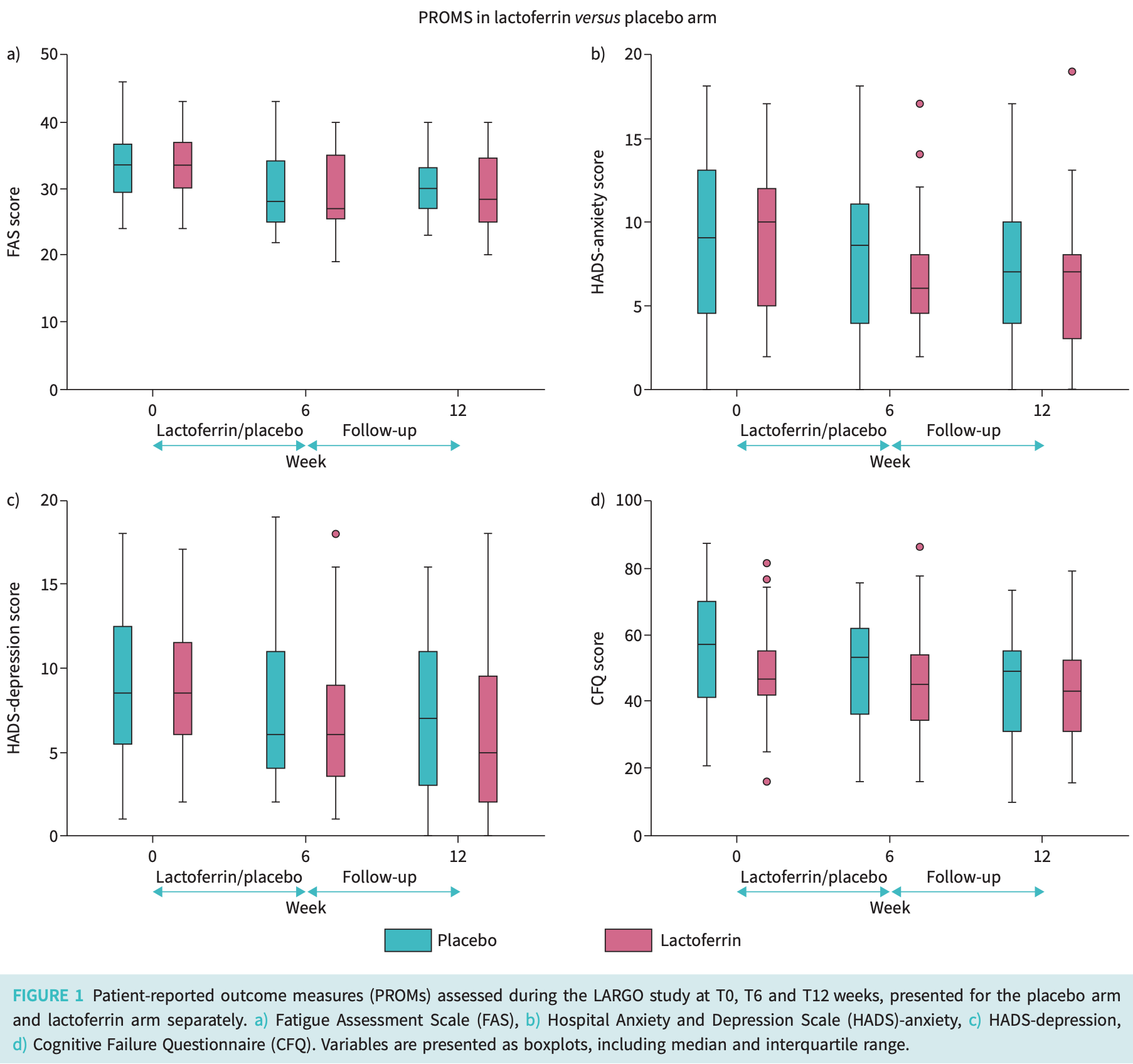
RCT 72 long COVID outpatients showing no significant difference in fatigue, anxiety, depression, or cognitive failure with 6 weeks of lactoferrin treatment compared to placebo.
Redel et al., 28 Mar 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Netherlands, peer-reviewed, median age 48.0, 5 authors, study period January 2022 - July 2022, LARGO trial.
Contact: a.redel2@franciscus.nl.
Effect of lactoferrin treatment on symptoms and physical performance in long COVID patients: a randomised, double-blind, placebo-controlled trial
doi:10.1183/23120541.00031-2024].
The randomised, double-blind, placebo-controlled LARGO trial investigated the effect of lactoferrin on long COVID symptoms. In both long COVID arms, clinical outcomes improved after 6 weeks without benefit for lactoferrin compared to placebo. https://bit.ly/3TvXHFC
Conflict of interest: A-L. Redel and F. Miry have nothing to disclose. M.E. Hellemons reports honoraria for lectures and consultancy from Boehringer Ingelheim, Pfizer and Takeda, unrelated to this study; and is an associate editor of this journal. L.M.A. Oswald reports honoraria for lectures from Stichting Medische Opleidingen. G.J. Braunstahl reports support for the present manuscript from Bonusan B.V.; honoraria for lectures and consultancy from GSK, AstraZeneca, Novartis and Sanofi Genzyme; and research grants from Sanofi Genzyme, GSK and AstraZeneca, not related to this study; and is Chairman of the NVALT Asthma Section, Secretary of the ERS Task Force on Allergy and Immunology and on the Scientific Advisory Board of Longfonds. Support statement: Bonusan B.V. contributed to this study by kindly providing the lactoferrin and placebo capsules. Bonusan B.V. had no influence on the study design, the execution of the study and the analysis of the study outcomes.
References
Appiah, Askie, Cramond, A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus
Barletta, Marino, Spagnolo, Coenzyme Q10+ɑ-lipoic acid for chronic COVID syndrome, Clin Exp Med
Bohannon, Muscle strength: clinical and prognostic value of hand-grip dynamometry, Curr Opin Clin Nutr Metab Care
Bolat, Eker, Kaplan, Lactoferrin for COVID-19 prevention, treatment, and recovery, Front Nutr
Buonsenso, Piazza, Boner, Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome, Allergy Asthma Proc
Burden, Long, Collaborators, Wulf Hanson, Abbafati, Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021, JAMA
Campione, Lanna, Cosio, Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence, Int J Environ Res Public Health
Ceban, Ling, Lui, Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis, Brain Behav Immun
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int J Antimicrob Agents
Davis, Mccorkell, Vogel, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
De Kleijn, Vries, Wijnen, Minimal (clinically) important differences for the Fatigue Assessment Scale in sarcoidosis, Respir Med
Drago-Serrano, Campos-Rodriguez, Carrero, Lactoferrin: balancing ups and downs of inflammation due to microbial infections, Int J Mol Sci
Geuter, Koban, Wager, The cognitive neuroscience of placebo effects: concepts, predictions, and physiology, Annu Rev Neurosci, doi:10.1183/23120541.00031-2024
Goertz, Van Herck, Delbressine, Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome?, ERJ Open Res
Hansen, Mogensen, Agergaard, High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial, Lancet Reg Health Eur
Hu, Meng, Zhang, The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor, Emerg Microbes Infect
Kruzel, Zimecki, Actor, Lactoferrin in a context of inflammation-induced pathology, Front Immunol
Langius, Visser, Kruizenga, Meetprotocol handknijpkracht m.b.v. Hand Dynamometer [Measurement protocol for hand grip strength using a hand dynanometer
Mccambridge, Witton, Elbourne, Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects, J Clin Epidemiol
Menges, Ballouz, Anagnostopoulos, Burden of post-COVID-19 syndrome and implications for healthcare service planning: a population-based cohort study, PLoS One
Ministerie Van Volksgezondheid, Welzijn en Sport. Varianten van het coronavirus SARS-CoV-2 [Variants of the coronavirus SARS-CoV-2
Muller, Benedetto, Aged brain and neuroimmune responses to COVID-19: post-acute sequelae and modulatory effects of behavioral and nutritional interventions, Immun Ageing
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med
Nopp, Moik, Klok, Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life, Respiration, doi:10.1183/23120541.00031-20248
Ponds, Van Boxtel, Jolles, De 'Cognitieve Failure Questionnaire' als maat voor subjectief cognitief functioneren [The 'Cognitive Failure Questionnaire' as measurement for subjective cognitive function, Tijdschrift voor neuropsychologie
Rathi, Jadhav, Shah, A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue, Medicines
Romanet, Wormser, Fels, Effectiveness of exercise training on the dyspnoea of individuals with long COVID: a randomised controlled multicentre trial, Ann Phys Rehabil Med
Schrimpf, Braesigk, Lippmann, Management and treatment of long COVID symptoms in general practices: an online-based survey, Front Public Health
Scragg, Jones, Fauvel, Psychological problems following ICU treatment, Anaesthesia
Singh, Pritam, Pandey, Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review, J Med Virol
Slotegraaf, Gerards, Verburg, Evaluation of primary allied health care in patients recovering from COVID-19 at 6-month follow-up: Dutch nationwide prospective cohort study, JMIR Public Health Surveill
Soriano, Murthy, Marshall, A clinical case definition of post-COVID-19 condition by a Delphi consensus, Lancet Infect Dis
Steinmetz, Gross, Lehnert, Longitudinal clinical features of post-COVID-19 patients-symptoms, fatigue and physical function at 3-and 6-month follow-up, J Clin Med
Tosato, Ciciarello, Zazzara, Nutraceuticals and dietary supplements for older adults with long COVID-19, Clin Geriatr Med
Wakabayashi, Oda, Yamauchi, Lactoferrin for prevention of common viral infections, J Infect Chemother
Yong, Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments, Infect Dis (Lond)
Zigmond, Snaith, The Hospital Anxiety and Depression Scale, Acta Psychiatr Scand
DOI record:
{
"DOI": "10.1183/23120541.00031-2024",
"ISSN": [
"2312-0541"
],
"URL": "http://dx.doi.org/10.1183/23120541.00031-2024",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Long COVID is a heterogeneous condition with a variety of symptoms that persist at least 3 months after SARS-CoV-2 infection, often with a profound impact on quality of life. Lactoferrin is an iron-binding glycoprotein with anti-inflammatory and antiviral properties. Current hypotheses regarding long COVID aetiology include ongoing immune activation, viral persistence and auto-immune dysregulation. Therefore, we hypothesised that long COVID patients may potentially benefit from lactoferrin treatment. The aims of the present study were to investigate the effect of lactoferrin on various long COVID domains: fatigue, anxiety, depression, cognitive failure and muscle strength.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We performed a randomised, double-blind, placebo-controlled trial in long COVID patients aged 18–70 years within 12 months after proven SARS-CoV-2 infection. Patients were randomised (1:1) to 6 weeks of lactoferrin (1200 mg daily) or placebo. At three hospital visits (T0, T6 and T12 weeks), patient-reported outcome measures were collected, physical performance tests were performed and blood was drawn. The difference in fatigue at T6 was the primary outcome.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>72 participants were randomised to lactoferrin (n=36) or placebo (n=36). We found a significant decrease in fatigue, as measured with the Fatigue Assessment Scale, between T0 and T6 in both study arms, but without significant difference between the study arms (lactoferrin: 3.9, 95% CI 2.3–5.5, p=0.007; placebo: 4.1, 95% CI 2.3–5.9, p=0.013). No significant differences were found in any of the other outcomes in favour of the lactoferrin arm at T6 or T12.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Although both long COVID arms showed improved clinical outcomes at T6, the improvement did not continue until T12. Lactoferrin provided no benefit in terms of fatigue, other patient-reported outcome measures or physical functioning.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2024,
3,
16
]
]
},
"alternative-id": [
"10.1183/23120541.00031-2024"
],
"author": [
{
"ORCID": "http://orcid.org/0009-0002-2990-9069",
"affiliation": [],
"authenticated-orcid": false,
"family": "Redel",
"given": "Anne-Lotte",
"sequence": "first"
},
{
"affiliation": [],
"family": "Miry",
"given": "Fatana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6666-5027",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hellemons",
"given": "Merel Elise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oswald",
"given": "Laurien Maria Amarentia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7671-3742",
"affiliation": [],
"authenticated-orcid": false,
"family": "Braunstahl",
"given": "Gerrit Johannes",
"sequence": "additional"
}
],
"container-title": "ERJ Open Research",
"container-title-short": "ERJ Open Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ersjournals.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T17:15:40Z",
"timestamp": 1711646140000
},
"deposited": {
"date-parts": [
[
2024,
7,
29
]
],
"date-time": "2024-07-29T08:50:49Z",
"timestamp": 1722243049000
},
"indexed": {
"date-parts": [
[
2024,
7,
30
]
],
"date-time": "2024-07-30T00:14:11Z",
"timestamp": 1722298451891
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
3,
28
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
7,
29
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
28
]
],
"date-time": "2024-03-28T00:00:00Z",
"timestamp": 1711584000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/23120541.00031-2024",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "00031-2024",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2024,
3,
28
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
28
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"DOI": "10.1371/journal.pone.0254523",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.1"
},
{
"DOI": "10.1016/S1473-3099(21)00703-9/ATTACHMENT/31179DEC-CF64-455B-BD13-6EF0045B7DD6/MMC1.PDF",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.2"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.3"
},
{
"DOI": "10.1080/23744235.2021.1924397",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.4"
},
{
"DOI": "10.1183/23120541.00542-2020",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.5"
},
{
"DOI": "10.1002/jmv.26254",
"article-title": "Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "J Med Virol",
"key": "2024072901500605000_10.4.00031-2024.6",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.2500/aap.2022.43.220018",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.7"
},
{
"DOI": "10.1186/s12979-023-00341-z",
"article-title": "Aged brain and neuroimmune responses to COVID-19: post-acute sequelae and modulatory effects of behavioral and nutritional interventions",
"author": "Muller",
"doi-asserted-by": "crossref",
"first-page": "17",
"journal-title": "Immun Ageing",
"key": "2024072901500605000_10.4.00031-2024.8",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.9"
},
{
"DOI": "10.1016/j.rehab.2023.101765",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.10"
},
{
"DOI": "10.3389/fpubh.2022.937100",
"article-title": "Management and treatment of long COVID symptoms in general practices: an online-based survey",
"author": "Schrimpf",
"doi-asserted-by": "crossref",
"first-page": "937100",
"journal-title": "Front Public Health",
"key": "2024072901500605000_10.4.00031-2024.11",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1159/000522118",
"article-title": "Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life",
"author": "Nopp",
"doi-asserted-by": "crossref",
"first-page": "593",
"journal-title": "Respiration",
"key": "2024072901500605000_10.4.00031-2024.12",
"volume": "101",
"year": "2022"
},
{
"DOI": "10.2196/44155",
"article-title": "Evaluation of primary allied health care in patients recovering from COVID-19 at 6-month follow-up: Dutch nationwide prospective cohort study",
"author": "Slotegraaf",
"doi-asserted-by": "crossref",
"first-page": "e44155",
"journal-title": "JMIR Public Health Surveill",
"key": "2024072901500605000_10.4.00031-2024.13",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.3390/ijms18030501",
"article-title": "Lactoferrin: balancing ups and downs of inflammation due to microbial infections",
"author": "Drago-Serrano",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Int J Mol Sci",
"key": "2024072901500605000_10.4.00031-2024.14",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.1016/j.jiac.2014.08.003",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.15"
},
{
"DOI": "10.3389/fimmu.2017.01438",
"article-title": "Lactoferrin in a context of inflammation-induced pathology",
"author": "Kruzel",
"doi-asserted-by": "crossref",
"first-page": "1438",
"journal-title": "Front Immunol",
"key": "2024072901500605000_10.4.00031-2024.16",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.3389/fnut.2022.992733",
"article-title": "Lactoferrin for COVID-19 prevention, treatment, and recovery",
"author": "Bolat",
"doi-asserted-by": "crossref",
"first-page": "992733",
"journal-title": "Front Nutr",
"key": "2024072901500605000_10.4.00031-2024.17",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2021.1888660",
"article-title": "The in vitro antiviral activity of lactoferrin against common human coronaviruses and SARS-CoV-2 is mediated by targeting the heparan sulfate co-receptor",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Emerg Microbes Infect",
"key": "2024072901500605000_10.4.00031-2024.18",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"article-title": "Lactoferrin as potential preventative and adjunct treatment for COVID-19",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "106118",
"journal-title": "Int J Antimicrob Agents",
"key": "2024072901500605000_10.4.00031-2024.19",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.3390/ijerph182010985",
"article-title": "Lactoferrin as antiviral treatment in COVID-19 management: preliminary evidence",
"author": "Campione",
"doi-asserted-by": "crossref",
"first-page": "10985",
"journal-title": "Int J Environ Res Public Health",
"key": "2024072901500605000_10.4.00031-2024.20",
"volume": "18",
"year": "2021"
},
{
"key": "2024072901500605000_10.4.00031-2024.21",
"unstructured": "Appiah JA , Askie L , Cramond V , et al. A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus. Geneva, World Health Organization, 2023."
},
{
"DOI": "10.1016/j.rmed.2011.05.004",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.22"
},
{
"DOI": "10.1097/MCO.0000000000000202",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.23"
},
{
"key": "2024072901500605000_10.4.00031-2024.24",
"unstructured": "Langius J , Visser W , Kruizenga H , et al. Meetprotocol handknijpkracht m.b.v. Hand Dynamometer [Measurement protocol for hand grip strength using a hand dynanometer]. Nutritional Assessment Platform, 2016. Available from: https://zakboekdietetiek.nl/wp-content/uploads/2016/04/Standard-Operating-Procedure-Handknijpkdracht-NAP.pdf Date last accessed: 16 May 2024."
},
{
"article-title": "De 'Cognitieve Failure Questionnaire' als maat voor subjectief cognitief functioneren [The 'Cognitive Failure Questionnaire' as measurement for subjective cognitive function]",
"author": "Ponds",
"first-page": "37",
"journal-title": "Tijdschrift voor neuropsychologie",
"key": "2024072901500605000_10.4.00031-2024.25",
"volume": "2",
"year": "2006"
},
{
"DOI": "10.1046/j.1365-2044.2001.01714.x",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.26"
},
{
"DOI": "10.1111/j.1600-0447.1983.tb09716.x",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.27"
},
{
"DOI": "10.1016/J.BBI.2021.12.020",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.28"
},
{
"DOI": "10.3390/jcm12123966",
"article-title": "Longitudinal clinical features of post-COVID-19 patients–symptoms, fatigue and physical function at 3- and 6-month follow-up",
"author": "Steinmetz",
"doi-asserted-by": "crossref",
"first-page": "3966",
"journal-title": "J Clin Med",
"key": "2024072901500605000_10.4.00031-2024.29",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3390/medicines8090047",
"article-title": "A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue",
"author": "Rathi",
"doi-asserted-by": "crossref",
"first-page": "47",
"journal-title": "Medicines (Basel)",
"key": "2024072901500605000_10.4.00031-2024.30",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1007/s10238-022-00871-8",
"article-title": "Coenzyme Q10+ɑ-lipoic acid for chronic COVID syndrome",
"author": "Barletta",
"doi-asserted-by": "crossref",
"first-page": "667",
"journal-title": "Clin Exp Med",
"key": "2024072901500605000_10.4.00031-2024.31",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.lanepe.2022.100539",
"article-title": "High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial",
"author": "Hansen",
"doi-asserted-by": "crossref",
"first-page": "100539",
"journal-title": "Lancet Reg Health Eur",
"key": "2024072901500605000_10.4.00031-2024.32",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.cger.2022.04.004",
"article-title": "Nutraceuticals and dietary supplements for older adults with long COVID-19",
"author": "Tosato",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Clin Geriatr Med",
"key": "2024072901500605000_10.4.00031-2024.33",
"volume": "38",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.18931",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.34"
},
{
"key": "2024072901500605000_10.4.00031-2024.35",
"unstructured": "Ministerie van Volksgezondheid, Welzijn en Sport . Varianten van het coronavirus SARS-CoV-2 [Variants of the coronavirus SARS-CoV-2]. Bilthoven, Rijksinstituut voor Volksgezondheid en Milieu (RIVM). https://www.rivm.nl/corona/actueel/virusvarianten#:∼:text=In%20oktober%202020%20werd%20in,als%20dominante%20virusvar iant%20in%20Nederland Date last accessed: 16 May 2024."
},
{
"DOI": "10.1016/j.jclinepi.2013.08.015",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.36"
},
{
"DOI": "10.1146/annurev-neuro-072116-031132",
"doi-asserted-by": "publisher",
"key": "2024072901500605000_10.4.00031-2024.37"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "http://openres.ersjournals.com/lookup/doi/10.1183/23120541.00031-2024"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of lactoferrin treatment on symptoms and physical performance in long COVID patients: a randomised, double-blind, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1183/ers-crossmark-policy",
"volume": "10"
}