Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19
et al., Journal of Virus Eradication, doi:10.1016/S2055-6640(20)30017-0, Apr 2020
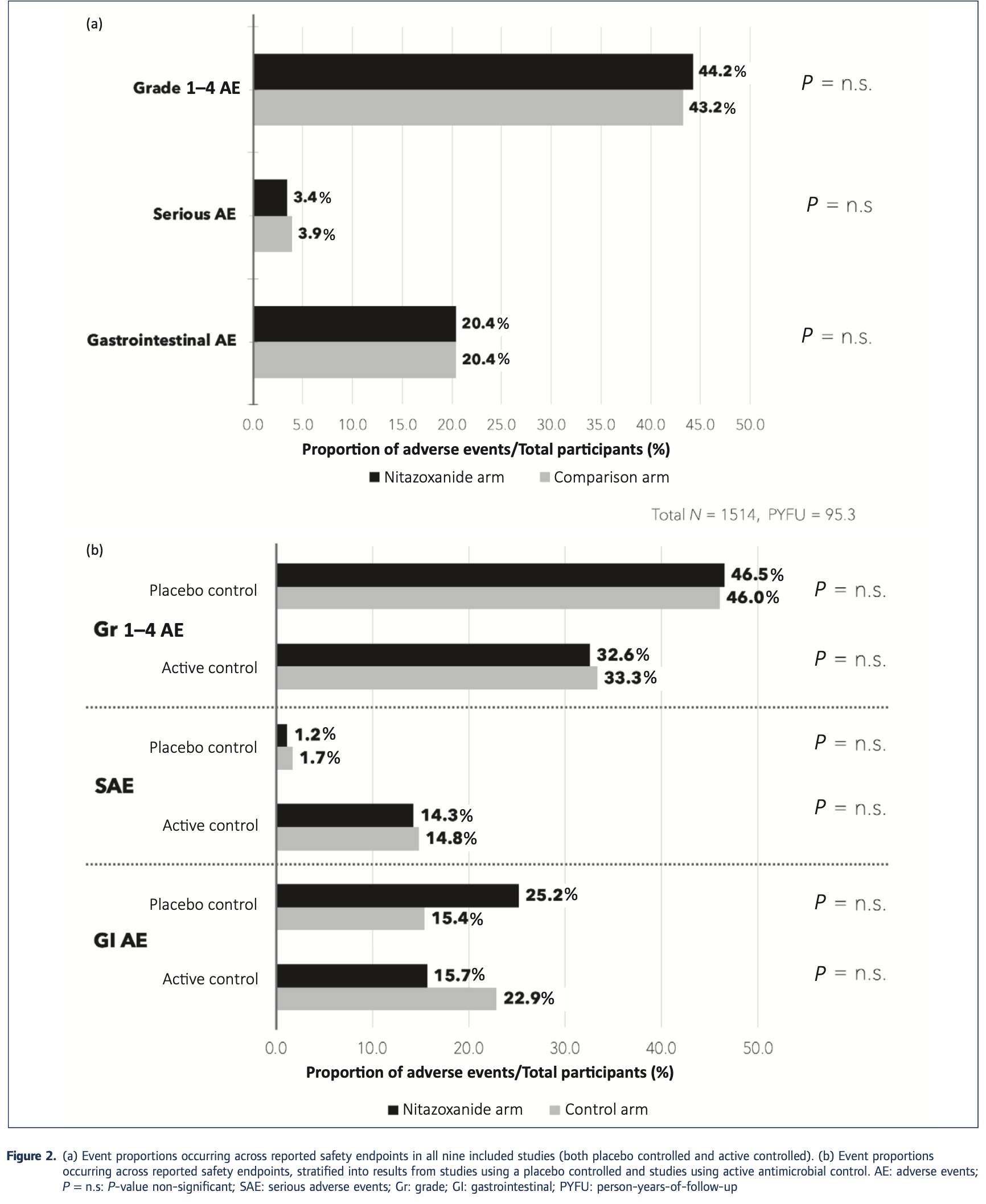
Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. Nine RCTs comparing nitazoxanide with placebo or active control for 5-14 days in participants experiencing acute infections were included, accounting for 1,514 participants and an estimated 95.3 person-years-of-follow-up. No significant differences were found in any of the adverse event endpoints assessed, across all trials or on subgroup analyses of active- or placebo-controlled trials. Mild gastrointestinal adverse events increased with dose. There were no teratogenic concerns, but the evidence base was very limited. Based on a weighted-mean cost of $61/kg, a 14-day course of treatment with nitazoxanide 500 mg twice daily would cost $1.41. The same 14-day course could cost $3944 in US commercial pharmacies, and $3 per course in Pakistan, India and Bangladesh. At a higher dose of 1100 mg three times daily, the estimated cost was $4.08 per 14-day course.
See Brechot et al. for another review covering nitazoxanide for COVID-19.
Pepperrell et al., 30 Apr 2020, peer-reviewed, 5 authors.
Contact: microhaart@aol.com.
Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19
Background: Many treatments are being assessed for repurposing to treat coronavirus disease 2019 . One drug that has shown promising results in vitro is nitazoxanide. Unlike other postulated drugs, nitazoxanide shows a high ratio of maximum plasma concentration (C max ), after 1 day of 500 mg twice daily (BD), to the concentration required to inhibit 50% replication (eC 50 ) of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) (C max : eC 50 roughly equal to 14:1). As such, it is important to investigate the safety of nitazoxanide for further trials. Furthermore, treatments for COviD-19 should be cheap to promote global access, but prices of many drugs are far higher than the costs of production. we aimed to conduct a review of the safety of nitazoxanide for any prior indication and calculate its minimum costs of production. Methods: A review of nitazoxanide clinical research was conducted using eMBASe and MeDLiNe databases, supplemented by ClinicalTrials.gov. we searched for phase 2 or 3 randomised controlled trials (RCTs) comparing nitazoxanide with placebo or active control for 5-14 days in participants experiencing acute infections of any kind. Data extracted were grade 1-4 and serious adverse events (Aes). Data were also extracted on gastrointestinal (Gi) Aes, as well as hepatorenal and cardiovascular effects. Active pharmaceutical ingredient cost data from 2016 to 2019 were extracted from the Panjiva database and adjusted for 5% loss during production, costs of excipients, formulation, a 10% profit margin and tax. Two dosages, at 500 mg BD and a higher dose of 1100 mg three times daily (TDS), were considered. Our estimated costs were compared with publicly available list prices from a selection of countries. Results: Nine RCTs of nitazoxanide were identified for inclusion. These RCTs accounted for 1514 participants and an estimated 95.3 person-years-of-follow-up. No significant differences were found in any of the Ae endpoints assessed, across all trials or on subgroup analyses of active-or placebo-controlled trials. Mild Gi Aes increased with dose. No hepatorenal or cardiovascular concerns were raised, but few appropriate metrics were reported. There were no teratogenic concerns, but the evidence base was very limited. Based on a weighted-mean cost of US $61/kg, a 14-day course of treatment with nitazoxanide 500 mg BD would cost $1.41. The daily cost would therefore be $0.10. The same 14-day course could cost $3944 in US commercial pharmacies, and $3 per course in Pakistan, india and Bangladesh. At a higher dose of 1100 mg TDS, our estimated cost was $4.08 per 14-day course, equivalent to $0.29 per day. Conclusion: Nitazoxanide demonstrates a good safety profile at approved doses. However, further evidence is required regarding hepatorenal and cardiovascular effects, as well as teratogenicity. we estimate that it would be possible to manufacture nitazoxanide as generic for $1.41 for a 14-day treatment course at 500 mg BD, up to..
Nitazoxanide safety and pricing review 57 most commonly used doses of 500 mg BD, with age and weight variability in paediatric dosing. Dose ranging studies were small and of shorter duration. if doses of >1000 mg per day are required for COviD-19 treatment, the possibility of dose-dependent Ae should be considered and further review will be required. The generalisability of these findings is limited to the settings and populations in which the included trials were carried out, with a large proportion of the participants from the included studies being young (mean age 37 years). This means findings may be less applicable to older COviD-19 patients.
Gastrointestinal effects Phase 1 studies of nitazoxanide identified Gi events as the most common side effect. These events were mild and no other serious events were noted [6, 20] . On literature review, many small studies for 3 days of nitazoxanide to treat diarrhoeal disease in Conflicts of interest vP and TP have no conflicts of interest to declare. AH received a consultancy payment from Merck for a clinical trial review that is not connected with this project. AO received grant funding and consultancy from Merck, viiv Healthcare and Gilead. He is a Director for Tandem Nano Ltd, which is not related to this project.
References
Alina®, Fda, Prescribing information
Arshad, Pertinez, Box, Prioritisation of potential anti-SARS-Cov-2 drug repurposing opportunities based on ability to achieve adequate target site concentrations derived from their established human pharmacokinetics, medRxiv, doi:10.1101/2020.04.16.20068379v1
Clerkin, Fried, Raikhelkar, Coronavirus disease 2019 (COviD-19) and cardiovascular disease circulation, Circulation, doi:10.1161/CiRCULATiO-NAHA.120.046941
Clinicaltrials, Gov, Study of nitazoxanide in adults with acute uncomplicated influenza
Cui, Chen, Li, Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia, J Thromb Haemost
Danzi, Loffi, Galeazzi, Acute pulmonary embolism and COviD-19 pneumonia: a random association?, Eur Heart J, doi:10.1093/eurheartj/ehaa254
Favennec, Ortiz, Gargala, Double-blind, randomized, placebocontrolled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru, Aliment Pharmacol Ther
Gamiño-Arroyo, Luerrero, Mccarthy, efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness, Clin Infect Dis
Gao, Li, Han, Diagnostic utility of clinical laboratory data determinations for patients with the severe COviD-19, J Med Virol, doi:10.1002/jmv.25770
Haffizulla, Hartman, Hoppers, effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial, Lancet Infect Dis
Hill, Barber, Gotham, estimated costs of production and potential prices for the wHO essential medicines list, BMJ Glob Health
Hill, Khoo, Fortunak, Minimum costs for producing hepatitis C directacting antivirals for use in large-scale treatment access programs in developing countries, Clin Infect Dis
Hill, Simmons, Gotham, Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C, J Virus Erad
Hill, Wang, Levi, Minimum costs to manufacture new treatments for COviD-19, J Virus Erad
Jasenosky, Cadena, Ce, The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits ebola virus, iScience
Lippi, Ej, D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis, Thromb Haemost, doi:10.1055/s-0040-1709650
Medex, A comprehensive online medicine index of Bangladesh
Medindia, Drug price of all the brand names
Moher, Liberati, Tetzlaff, Preferred reporting items for systematic reviews and meta-analyses: the PRiSMA statement, PLoS Med
Montaner, Hill, Acosta E, Practical implications for the interpretation of minimum plasma concentration/inhibitory concentration ratios, Lancet
Musher, Logan, Bressler, Nitazoxanide versus vancomycin in clostridium difficile infection: a randomized, double-blind study, Clin Infect Dis
Musher, Logan, Hamill, Nitazoxanide for the treatment of Clostridium difficile colitis, Clin Infect Dis
Ong, Be, Ong, COviD-19 in gastroenterology: a clinical perspective, Gut, doi:10.1136/gutjnl-2020-321051
Padmanabhan, Potential dual therapeutic approach against SARS-Cov-2/COviD-19 with nitazoxanide and hydroxychloroquine, doi:10.13140/RG.2.2.28124.74882
Panjiva, Global trade insights
Pharmacy, None
Rossignol, Frazia, Chiappa, Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level, J Biol Chem
Rossignol, Kabil, El-Gohary, Nitazoxanide in the treatment of amoebiasis, Trans R Soc Trop Med Hyg
Rossignol, Nitazoxanide, a new drug candidate for the treatment of Middle east respiratory syndrome coronavirus, J Infect Public Health
Ruan, Yang, Wang, Clinical predictors of mortality due to COviD-19 based on an analysis of data of 150 patients from wuhan, China, Intensive Care Med, doi:10.1007/s00134-020-05991-x
Sanders, Monogue, Jodlowski, Pharmacologic treatments for coronavirus disease 2019 (COviD-19): a review, JAMA, doi:10.1001/jama.2020.6019
Shehata, Talaat, Soliman, Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection, Helicobacter
Song, Shin, COviD-19, a clinical syndrome manifesting as hypersensitivity pneumonitis, Infect Chemother
Stockis, Allemon, Bruyn, Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses, Int J Clin Pharmacol Ther
Stockis, De Bruyn, Gengler, Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5 g and 1 g b.i.d, Int J Clin Pharmacol Ther
Täubel, Lorch, Rossignol, Analyzing the relationship of QT interval and exposure to nitazoxanide, a prospective candidate for influenza antiviral therapy -a formal TQT study, J Clin Pharmacol
Vigiaccess, None
Walsh, Mcaulay, Lee, early bactericidal activity trial of nitazoxanide for pulmonary, Antimicrob Agents Chemother
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCov) in vitro, Cell Res
Yang, Sun, Chen, Diagnosis and treatment of COviD-19: acute kidney injury cannot be ignored, Zhonghua Yi Xue Za Zhi
Zheng, Ma, Zhang, COviD-19 and the cardiovascular system, Nat Rev Cardiol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COviD-19 in wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1016/s2055-6640(20)30017-0",
"ISSN": [
"2055-6640"
],
"URL": "http://dx.doi.org/10.1016/S2055-6640(20)30017-0",
"alternative-id": [
"S2055664020300170"
],
"author": [
{
"affiliation": [],
"family": "Pepperrell",
"given": "Toby",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pilkington",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Junzheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hill",
"given": "Andrew M.",
"sequence": "additional"
}
],
"container-title": "Journal of Virus Eradication",
"container-title-short": "Journal of Virus Eradication",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
2
]
],
"date-time": "2020-07-02T15:39:23Z",
"timestamp": 1593704363000
},
"deposited": {
"date-parts": [
[
2021,
3,
22
]
],
"date-time": "2021-03-22T20:47:07Z",
"timestamp": 1616446027000
},
"indexed": {
"date-parts": [
[
2024,
8,
1
]
],
"date-time": "2024-08-01T02:15:28Z",
"timestamp": 1722478528535
},
"is-referenced-by-count": 36,
"issue": "2",
"issued": {
"date-parts": [
[
2020,
4
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2020,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
4,
1
]
],
"date-time": "2020-04-01T00:00:00Z",
"timestamp": 1585699200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 92,
"start": {
"date-parts": [
[
2020,
7,
2
]
],
"date-time": "2020-07-02T00:00:00Z",
"timestamp": 1593648000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2055664020300170?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2055664020300170?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "52-60",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
4
]
]
},
"published-print": {
"date-parts": [
[
2020,
4
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"author": "US Food and Drug Agency",
"journal-title": "Fact sheet for health care providers emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients",
"key": "10.1016/S2055-6640(20)30017-0_bb0010",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review",
"author": "Sanders",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/S2055-6640(20)30017-0_bb0015",
"year": "2020"
},
{
"author": "Padmanabhan",
"journal-title": "Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with nitazoxanide and hydroxychloroquine",
"key": "10.1016/S2055-6640(20)30017-0_bb0020",
"year": "2020"
},
{
"DOI": "10.1016/j.jiph.2016.04.001",
"article-title": "Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "227",
"issue": "3",
"journal-title": "J Infect Public Health",
"key": "10.1016/S2055-6640(20)30017-0_bb0025",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.5414/CPP40213",
"article-title": "Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses",
"author": "Stockis",
"doi-asserted-by": "crossref",
"first-page": "213",
"issue": "5",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "10.1016/S2055-6640(20)30017-0_bb0030",
"volume": "40",
"year": "2002"
},
{
"DOI": "10.5414/CPP40221",
"article-title": "Nitazoxanide pharmacokinetics and tolerability in man during 7 days dosing with 0.5g and 1 g b.i.d",
"author": "Stockis",
"doi-asserted-by": "crossref",
"first-page": "221",
"issue": "5",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "10.1016/S2055-6640(20)30017-0_bb0035",
"volume": "40",
"year": "2002"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res",
"key": "10.1016/S2055-6640(20)30017-0_bb0040",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.16.20068379",
"doi-asserted-by": "crossref",
"key": "10.1016/S2055-6640(20)30017-0_bb0045",
"unstructured": "UArshad, HPertinez, HBoxet al.Prioritisation of potential anti-SARS-CoV-2 drug repurposing opportunities based on ability to achieve adequate target site concentrations derived from their established human pharmacokinetics. medRxiv22 April 2020. Available at: www.medrxiv.org/content/10.1101/2020.04.16.20068379v1 (accessed April 2020)."
},
{
"DOI": "10.1074/jbc.M109.029470",
"article-title": "Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "29798",
"issue": "43",
"journal-title": "J Biol Chem",
"key": "10.1016/S2055-6640(20)30017-0_bb0050",
"volume": "284",
"year": "2009"
},
{
"DOI": "10.1016/S0140-6736(00)04577-3",
"article-title": "Practical implications for the interpretation of minimum plasma concentration/inhibitory concentration ratios",
"author": "Montaner",
"doi-asserted-by": "crossref",
"first-page": "1438",
"issue": "9266",
"journal-title": "Lancet",
"key": "10.1016/S2055-6640(20)30017-0_bb0055",
"volume": "357",
"year": "2001"
},
{
"DOI": "10.3947/ic.2020.52.1.110",
"article-title": "COVID-19, a clinical syndrome manifesting as hypersensitivity pneumonitis",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "110",
"issue": "1",
"journal-title": "Infect Chemother",
"key": "10.1016/S2055-6640(20)30017-0_bb0060",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1016/j.isci.2019.07.003",
"article-title": "The FDA-approved oral drug nitazoxanide amplifies host antiviral responses and inhibits Ebola virus",
"author": "Jasenosky",
"doi-asserted-by": "crossref",
"first-page": "1279",
"journal-title": "iScience",
"key": "10.1016/S2055-6640(20)30017-0_bb0065",
"volume": "19",
"year": "2019"
},
{
"author": "FDA",
"key": "10.1016/S2055-6640(20)30017-0_bb0070",
"series-title": "Approval of nitazoxanide",
"year": "2004"
},
{
"DOI": "10.1016/S2055-6640(20)30018-2",
"article-title": "Minimum costs to manufacture new treatments for COVID-19",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "61",
"issue": "2",
"journal-title": "J Virus Erad",
"key": "10.1016/S2055-6640(20)30017-0_bb0075",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/S2055-6640(20)30691-9",
"article-title": "Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "28",
"issue": "1",
"journal-title": "J Virus Erad",
"key": "10.1016/S2055-6640(20)30017-0_bb0080",
"volume": "2",
"year": "2016"
},
{
"DOI": "10.1136/bmjgh-2017-000571",
"article-title": "Estimated costs of production and potential prices for the WHO essential medicines list",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "e000571",
"issue": "1",
"journal-title": "BMJ Glob Health",
"key": "10.1016/S2055-6640(20)30017-0_bb0085",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1093/cid/ciu012",
"article-title": "Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries",
"author": "Hill",
"doi-asserted-by": "crossref",
"first-page": "928",
"issue": "7",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2055-6640(20)30017-0_bb0090",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1371/journal.pmed.1000097",
"article-title": "Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement",
"author": "Moher",
"doi-asserted-by": "crossref",
"first-page": "e1000097",
"issue": "7",
"journal-title": "PLoS Med",
"key": "10.1016/S2055-6640(20)30017-0_bb0095",
"volume": "6",
"year": "2009"
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0100",
"unstructured": "Alinia ® (nitazoxanide) tablets (nitazoxanide) for oral suspension Available atwww.alinia.com/healthcare-professionals/alinia-nitazoxanide-500-mg-tablets/accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0105",
"unstructured": "Prescribing information; Alina®. FDA, 2005www.accessdata.fda.gov/drugsatfda_docs/label/2005/021818lbl.pdfaccessed April 2020)."
},
{
"DOI": "10.1136/gutjnl-2020-321051",
"article-title": "COVID-19 in gastroenterology: a clinical perspective",
"author": "Ong",
"doi-asserted-by": "crossref",
"journal-title": "Gut",
"key": "10.1016/S2055-6640(20)30017-0_bb0110",
"year": "2020"
},
{
"article-title": "Diagnosis and treatment of COVID-19: acute kidney injury cannot be ignored",
"author": "Yang",
"journal-title": "Zhonghua Yi Xue Za Zhi",
"key": "10.1016/S2055-6640(20)30017-0_bb0115",
"volume": "100",
"year": "2020"
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0120",
"unstructured": "Panjiva. Global trade insights. Available at:www.panjiva.com (accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0125",
"unstructured": "Drugs.com. Drug price information. Available at:www.drugs.com/price-guide/."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0130",
"unstructured": "US Department of Veterans Affairs. Office of Procurement, Acquisitions and Logistics. Pharmaceutical Prices 15 March 2020. Available at:www.va.gov/opal/nac/fss/pharmPrices.asp (accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0135",
"unstructured": "Brazilian Health Regulatory Agency. Drug price lists. Available at:portal.anvisa.gov.br/listas-de-precos (accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0140",
"unstructured": "MedIndia. Drug price of all the brand names. Available at:www.medindia.net/drug-price/index.asp (accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0145",
"unstructured": "MedEx. A comprehensive online medicine index of Bangladesh. Available at:www.medindia.net/drug-price/index.asp (accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0150",
"unstructured": "Sehat PharmacyAvailable at:sehat.com.pk/accessed April 2020)."
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0155",
"unstructured": "Egyptian Drug StoreAvailable at:egyptiandrugstore.com/index.php?route=common/homeaccessed April 2020)."
},
{
"DOI": "10.1086/506351",
"article-title": "Nitazoxanide for the treatment of Clostridium difficile colitis",
"author": "Musher",
"doi-asserted-by": "crossref",
"first-page": "421",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2055-6640(20)30017-0_bb0160",
"volume": "43",
"year": "2006"
},
{
"DOI": "10.1111/hel.12395",
"article-title": "Randomized controlled study of a novel triple nitazoxanide (NTZ)-containing therapeutic regimen versus the traditional regimen for eradication of Helicobacter pylori infection",
"author": "Shehata",
"doi-asserted-by": "crossref",
"first-page": "e12395",
"issue": "5",
"journal-title": "Helicobacter",
"key": "10.1016/S2055-6640(20)30017-0_bb0165",
"volume": "22",
"year": "2017"
},
{
"DOI": "10.1086/596552",
"article-title": "Nitazoxanide versus vancomycin in clostridium difficile infection: a randomized, double-blind study",
"author": "Musher",
"doi-asserted-by": "crossref",
"first-page": "e41",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2055-6640(20)30017-0_bb0170",
"volume": "48",
"year": "2009"
},
{
"DOI": "10.1128/AAC.01956-19",
"article-title": "Early bactericidal activity trial of nitazoxanide for pulmonary",
"author": "Walsh",
"doi-asserted-by": "crossref",
"first-page": "e01956",
"issue": "5",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/S2055-6640(20)30017-0_bb0175",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(14)70717-0",
"article-title": "Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase 2b/3 trial",
"author": "Haffizulla",
"doi-asserted-by": "crossref",
"first-page": "609",
"issue": "7",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S2055-6640(20)30017-0_bb0180",
"volume": "14",
"year": "2014"
},
{
"DOI": "10.1093/cid/ciz100",
"article-title": "Efficacy and safety of nitazoxanide in addition to standard of care for the treatment of severe acute respiratory illness",
"author": "Gamiño-Arroyo",
"doi-asserted-by": "crossref",
"first-page": "1903",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/S2055-6640(20)30017-0_bb0185",
"volume": "69",
"year": "2019"
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0190",
"unstructured": "ClinicalTrials.gov. Study of nitazoxanide in adults with acute uncomplicated influenza. Identifier: NCT01056380 Bethesda (MD): US National Library of Medicine. 2010 January 26. Available at:clinicaltrials.gov/ct2/show/NCT01056380 (accessed April 2020)."
},
{
"DOI": "10.1046/j.1365-2036.2003.01419.x",
"article-title": "Double-blind, randomized, placebo-controlled study of nitazoxanide in the treatment of fascioliasis in adults and children from northern Peru",
"author": "Favennec",
"doi-asserted-by": "crossref",
"first-page": "265",
"issue": "2",
"journal-title": "Aliment Pharmacol Ther",
"key": "10.1016/S2055-6640(20)30017-0_bb0195",
"volume": "17",
"year": "2003"
},
{
"DOI": "10.1016/j.trstmh.2007.04.001",
"article-title": "Nitazoxanide in the treatment of amoebiasis",
"author": "Rossignol",
"doi-asserted-by": "crossref",
"first-page": "1025",
"issue": "10",
"journal-title": "Trans R Soc Trop Med Hyg",
"key": "10.1016/S2055-6640(20)30017-0_bb0200",
"volume": "101",
"year": "2007"
},
{
"key": "10.1016/S2055-6640(20)30017-0_bb0205",
"unstructured": "VigiAccessAvailable at:www.vigiaccess.org/accessed April 2020)."
},
{
"DOI": "10.1002/jcph.300",
"article-title": "Analyzing the relationship of QT interval and exposure to nitazoxanide, a prospective candidate for influenza antiviral therapy – a formal TQT study",
"author": "Täubel",
"doi-asserted-by": "crossref",
"first-page": "987",
"issue": "9",
"journal-title": "J Clin Pharmacol",
"key": "10.1016/S2055-6640(20)30017-0_bb0210",
"volume": "54",
"year": "2014"
},
{
"article-title": "Coronavirus disease 2019 (COVID-19) and cardiovascular disease circulation",
"author": "Clerkin",
"journal-title": "Circulation",
"key": "10.1016/S2055-6640(20)30017-0_bb0215",
"year": "2020"
},
{
"DOI": "10.1038/s41569-020-0360-5",
"article-title": "COVID-19 and the cardiovascular system",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "259",
"issue": "5",
"journal-title": "Nat Rev Cardiol",
"key": "10.1016/S2055-6640(20)30017-0_bb0220",
"volume": "17",
"year": "2020"
},
{
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China",
"author": "Ruan",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2055-6640(20)30017-0_bb0225",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "10.1016/S2055-6640(20)30017-0_bb0230",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1111/jth.14830",
"article-title": "Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia",
"author": "Cui",
"doi-asserted-by": "crossref",
"journal-title": "J Thromb Haemost",
"key": "10.1016/S2055-6640(20)30017-0_bb0235",
"year": "2020"
},
{
"DOI": "10.1093/eurheartj/ehaa254",
"article-title": "Acute pulmonary embolism and COVID-19 pneumonia: a random association?",
"author": "Danzi",
"doi-asserted-by": "crossref",
"journal-title": "Eur Heart J",
"key": "10.1016/S2055-6640(20)30017-0_bb0240",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25770",
"article-title": "Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19",
"author": "Gao",
"doi-asserted-by": "crossref",
"journal-title": "J Med Virol",
"key": "10.1016/S2055-6640(20)30017-0_bb0245",
"year": "2020"
},
{
"article-title": "D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis",
"author": "Lippi",
"journal-title": "Thromb Haemost",
"key": "10.1016/S2055-6640(20)30017-0_bb0250",
"year": "2020"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2055664020300170"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19",
"type": "journal-article",
"volume": "6"
}