Apr 1 2023 |
et al., NCT05187793 | A Multicenter, Open-label, Randomized Study of the Efficacy and Safety of Artlegia (INN: Olokizumab) New Dosing Regimen in Patients With Coronavirus Infection (COVID-19) With Signs of Hyperinflammation |
| 198 patient olokizumab late treatment RCT with results not reported over 2 years after completion. | ||
Aug 29 2022 |
et al., NCT05196477 | Multicentre Non-interventional Retrospective Cohort Study of the Outcomes of Olokizumab Therapy in Hospitalized Patients With SARS-CoV-2 (COVID-19) Infection |
| 3,087 patient olokizumab late treatment study with results not reported over 3 years after completion. | ||
Jan 24 2022 |
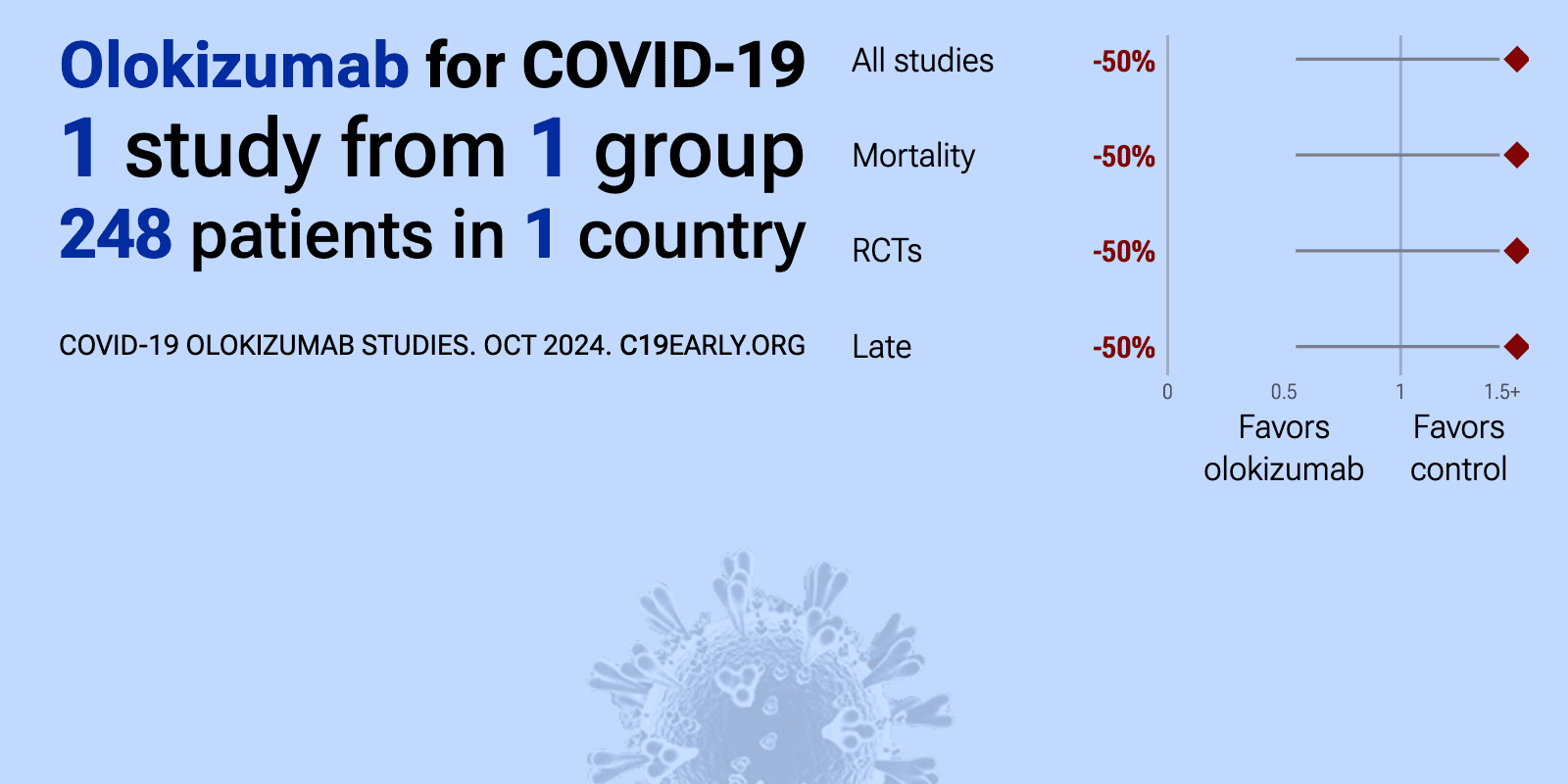
et al., NCT04380519 | An International, Multicenter, Randomized, Double-blind, Adaptive Placebo-controlled Study of the Efficacy and Safety of a Single Administration of Olokizumab and RPH-104 With Standard Therapy in Patients With Severe SARS-CoV-2 Infection (COVID-19) |
| 50% higher mortality (p=0.6) and 30% greater improvement (p=0.21). RCT 248 hospitalized patients showing no significant differences with olokuzimab. | ||