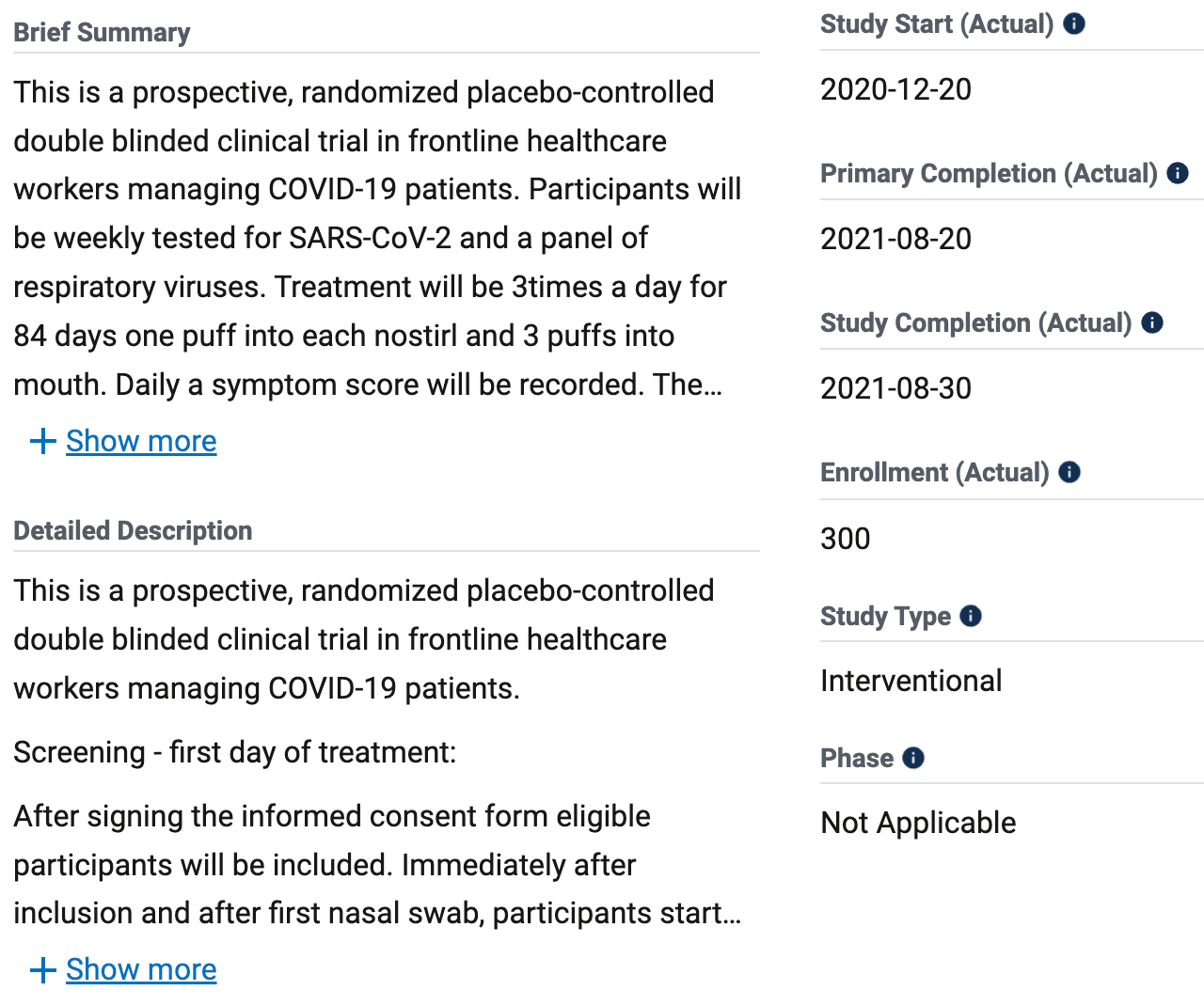
Clinical Trial to Evaluate the Efficacy of an Iota-Carrageenan Nasal Spray to Reduce Symptoms Caused by SARS-CoV-2 and Other Respiratory Viruses in Healthcare Workers Managing COVID-19 Patients
et al., NCT04681001, NCT04681001, Aug 2021
300 participant iota-carrageenan prophylaxis RCT with results not reported over 4 years after completion.
Authors indicate that there were no SARS-CoV-2 infections in either group. The trial record also suggests there was no placebo group, with both groups receiving a nasal spray.
Niculescu et al., 30 Aug 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Austria, trial NCT04681001 (history).
Contact: iulia.niculescu@gesundheitsverbund.at, eva.prieschl-grassauer@marinomed.com, nicole.unger-manhart@marinomed.com, arschang.valipour@gesundheitsverbund.at, waltraud.huf@gesundheitsverbund.at.