Payments by Drug and Medical Device Manufacturers to US Peer Reviewers of Major Medical Journals
et al., JAMA, doi:10.1001/jama.2024.17681, Oct 2024
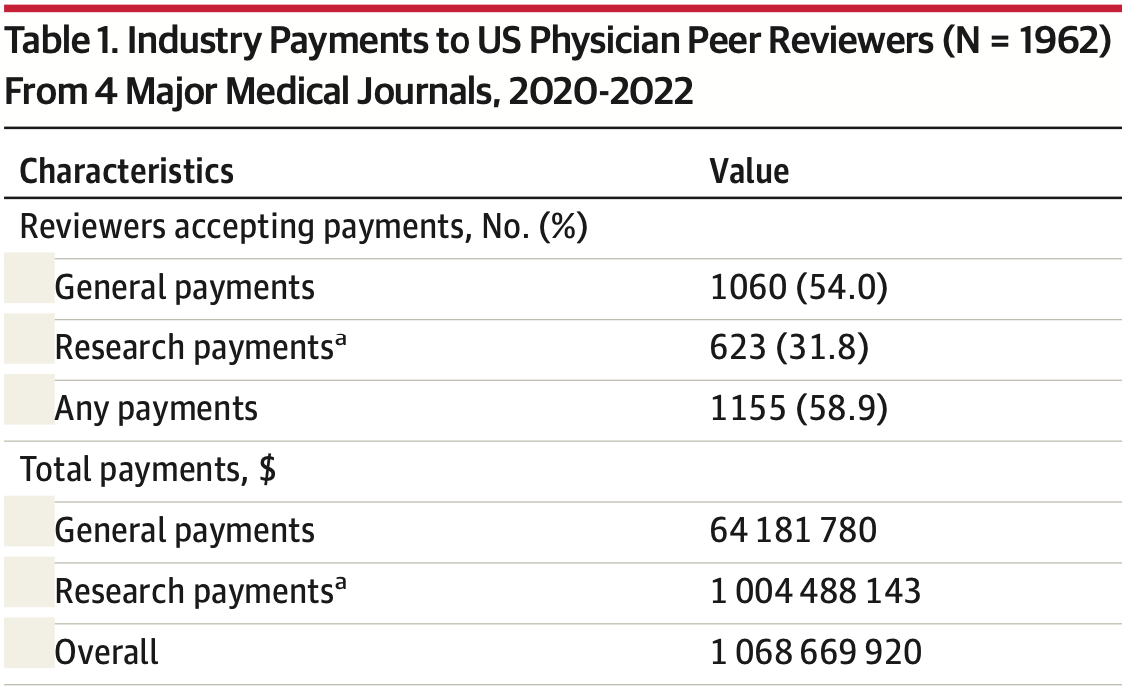
Analysis of payments by drug and medical device manufacturers to US physician peer reviewers of 4 major medical journals from 2020-2022. Authors found that 59% of 1,962 eligible reviewers received industry payments totaling over $US1 billion, with a median payment of $US 31,254 per physician.
Nguyen et al., 10 Oct 2024, Canada, peer-reviewed, 7 authors.
Contact: wallis.cjd@gmail.com.
Abstract: Letters
RESEARCH LETTER
Payments by Drug and Medical Device
Manufacturers to US Peer Reviewers
of Major Medical Journals
Table 1. Industry Payments to US Physician Peer Reviewers (N = 1962)
From 4 Major Medical Journals, 2020-2022
Characteristics
Although conflicts of interest of journal editors and authors
have been investigated,1,2 the traditionally opaque nature of
peer review has hindered their evaluation among peer reviewers, despite their crucial role in academic publishing. While
most journals have established conflict of interest
Supplemental content
policies for authors, fewer extend these policies to peer reviewers.3 In many cases, journals or editors may inquire about reviewer conflicts of interest and consider these while managing the peer review process,
although publicly available reviewer conflict of interest disclosures are rare. Reviewers of leading medical journals may
have industry ties due to their academic expertise.
We sought to characterize payments by drug and medical
device manufacturers to US peer reviewers of major medical journals.
Methods | We identified peer reviewers for The BMJ, JAMA,
The Lancet, and The New England Journal of Medicine (NEJM)
using each journal’s 2022 reviewer list. These journals were selected for their high impact factor and reputation as leading
publications of original general medical research. Because reviewer lists did not include affiliations, identification was conducted using Scopus and the National Plan and Provider
Enumeration System, which also provided sex and specialty
information. We limited our cohort to US-based physicians due
to use of the Centers for Medicare & Medicaid Services Open
Payments database. Two independent abstractors (A.-L.N.,
L.M.) performed the search strategy for each reviewer, with discrepancies resolved by a third author (D.-D.N.).
We extracted general and research payments to the identified peer reviewers between 2020 and 2022 from the Open
Payments database, capturing payments from drug and medical device manufacturers to US-licensed physicians.4 We excluded ownership and investment interests because they are
not equivalent to financial transfers and are less reliable than
other general payments. Research payments included payments to individual physicians and institutional payments for
research where they served as principal investigators. Institutional payments were divided by the number of principal investigators. Inflation-adjusted payment amounts in 2022
US dollars were calculated among those receiving payments.
We compared industry payments by sex and specialty using
the Mann-Whitney U test and Kruskal-Wallis test with subsequent Dunn pairwise testing accounting for multiple testing,
respectively. Analyses were performed using Stata MP version 17.0 (StataCorp). Statistical significance was defined as
a 2-sided P < .05.
jama.com
Value
Reviewers accepting payments, No. (%)
General payments
1060 (54.0)
Research paymentsa
623 (31.8)
Any payments
1155 (58.9)
Total payments, $
General payments
64 181 780
Research paymentsa
1 004 488 143
Overall
1 068 669 920
Payment received per physician,
median (IQR), $b
General payments
7614 (495-43 069)
Research paymentsa
153 173 (29 307-835 637)
Overall
31 254 (2000-276 958)
Total payments by category, $ (%)
Research payments via institutions
(associated research funding)c
999 197 635 (93.5)
Consulting fees
34 313 903 (3.2)
Speaking compensation not related
to CME program
11 801 491..
DOI record:
{
"DOI": "10.1001/jama.2024.17681",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2024.17681",
"abstract": "<jats:p>This study characterizes payments by drug and medical device manufacturers to US peer reviewers of major medical journals.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Division of Urology, University of Toronto, Toronto, Ontario, Canada"
},
{
"name": "Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, Ontario, Canada"
}
],
"family": "Nguyen",
"given": "David-Dan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Medicine, Tohoku University, Sendai City, Miyagi, Japan"
},
{
"name": "Department of Population Health Science and Policy, Icahn School of Medicine at Mount Sinai, New York, New York"
}
],
"family": "Muramaya",
"given": "Anju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Schulich School of Medicine and Dentistry, Western University, Windsor, Ontario, Canada"
}
],
"family": "Nguyen",
"given": "Anna-Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada"
}
],
"family": "Cheng",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine and Health Sciences, McGill University, Montreal, Quebec, Canada"
}
],
"family": "Murad",
"given": "Liam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology, Houston Methodist Hospital, Houston, Texas"
}
],
"family": "Satkunasivam",
"given": "Raj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Urology, University of Toronto, Toronto, Ontario, Canada"
},
{
"name": "Division of Urology, Mount Sinai Hospital, Toronto, Ontario, Canada"
}
],
"family": "Wallis",
"given": "Christopher J. D.",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
10
]
],
"date-time": "2024-10-10T15:02:21Z",
"timestamp": 1728572541000
},
"deposited": {
"date-parts": [
[
2024,
10,
10
]
],
"date-time": "2024-10-10T15:02:24Z",
"timestamp": 1728572544000
},
"indexed": {
"date-parts": [
[
2024,
10,
11
]
],
"date-time": "2024-10-11T04:20:15Z",
"timestamp": 1728620415226
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
10,
10
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2824834/jama_nguyen_2024_ld_240062_1727972553.95638.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2024,
10,
10
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
10
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1136/bmj.j4619",
"article-title": "Payments by US pharmaceutical and medical device manufacturers to US medical journal editors: retrospective observational study.",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "j4619",
"journal-title": "BMJ",
"key": "jld240062r1",
"volume": "359",
"year": "2017"
},
{
"DOI": "10.1136/bmjopen-2021-057598",
"article-title": "A cross-sectional examination of conflict-of-interest disclosures of physician-authors publishing in high-impact US medical journals.",
"author": "Baraldi",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "BMJ Open",
"key": "jld240062r2",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1111/j.1525-1497.2006.00598.x",
"article-title": "Conflict of interest disclosure policies and practices in peer-reviewed biomedical journals.",
"author": "Cooper",
"doi-asserted-by": "publisher",
"first-page": "1248",
"issue": "12",
"journal-title": "J Gen Intern Med",
"key": "jld240062r3",
"volume": "21",
"year": "2006"
},
{
"DOI": "10.1001/jama.2020.11413",
"article-title": "Trends in industry payments to physicians in the United States from 2014 to 2018.",
"author": "Marshall",
"doi-asserted-by": "publisher",
"first-page": "1785",
"issue": "17",
"journal-title": "JAMA",
"key": "jld240062r5",
"volume": "324",
"year": "2020"
},
{
"key": "jld240062r4",
"unstructured": "Centers for Medicare & Medicaid Services. Open Payments. Accessed June 1, 2024. https://openpaymentsdata.cms.gov/"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2824834"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Payments by Drug and Medical Device Manufacturers to US Peer Reviewers of Major Medical Journals",
"type": "journal-article"
}