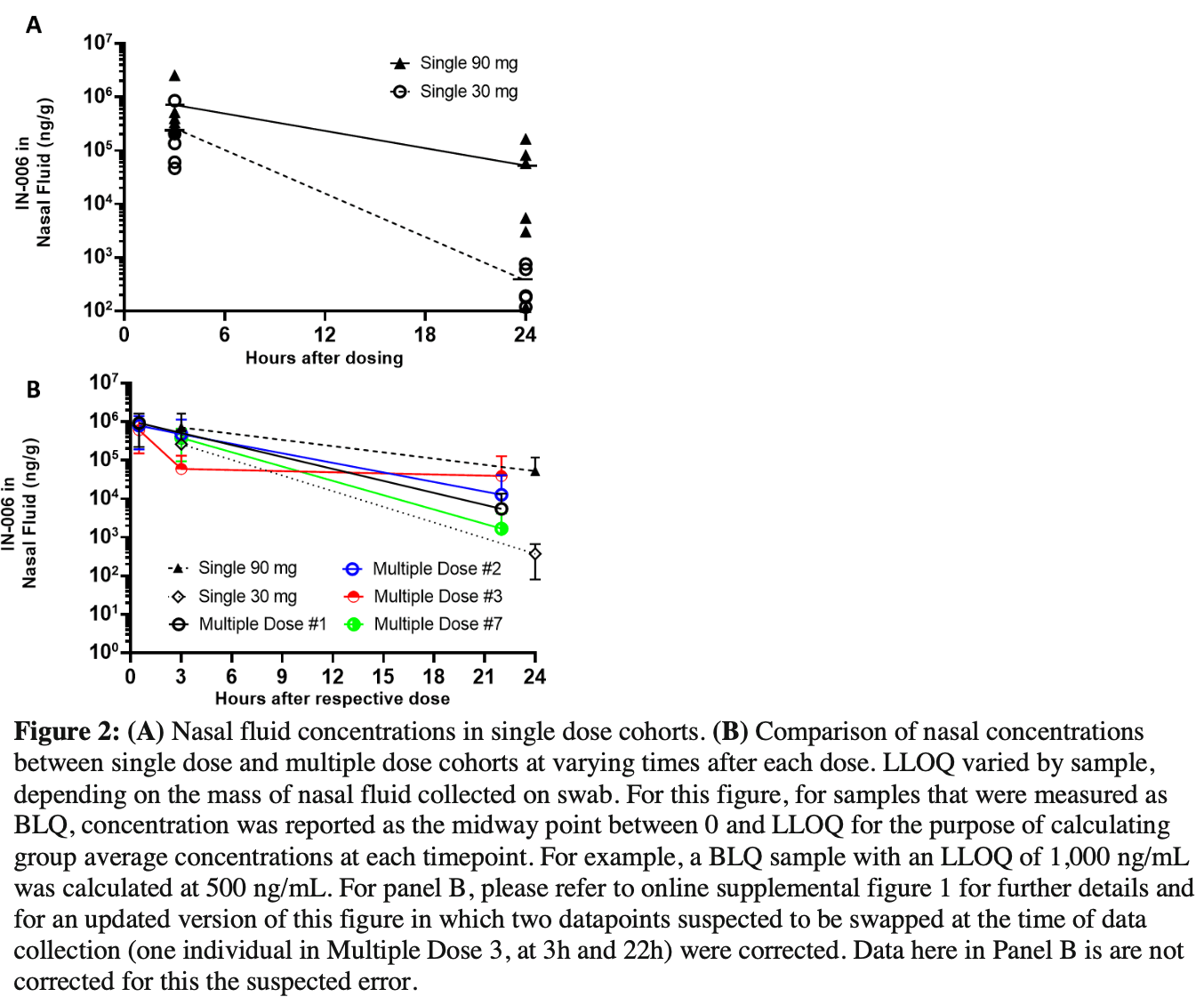
A randomized, double-blind, Phase 1, single- and multiple-dose placebo-controlled study of the safety and pharmacokinetics of IN-006, an inhaled antibody treatment for COVID-19 in healthy volunteers
et al., eBioMedicine, doi:10.1016/j.ebiom.2025.105582, ACTRN12621001235897, Aug 2022 (preprint)
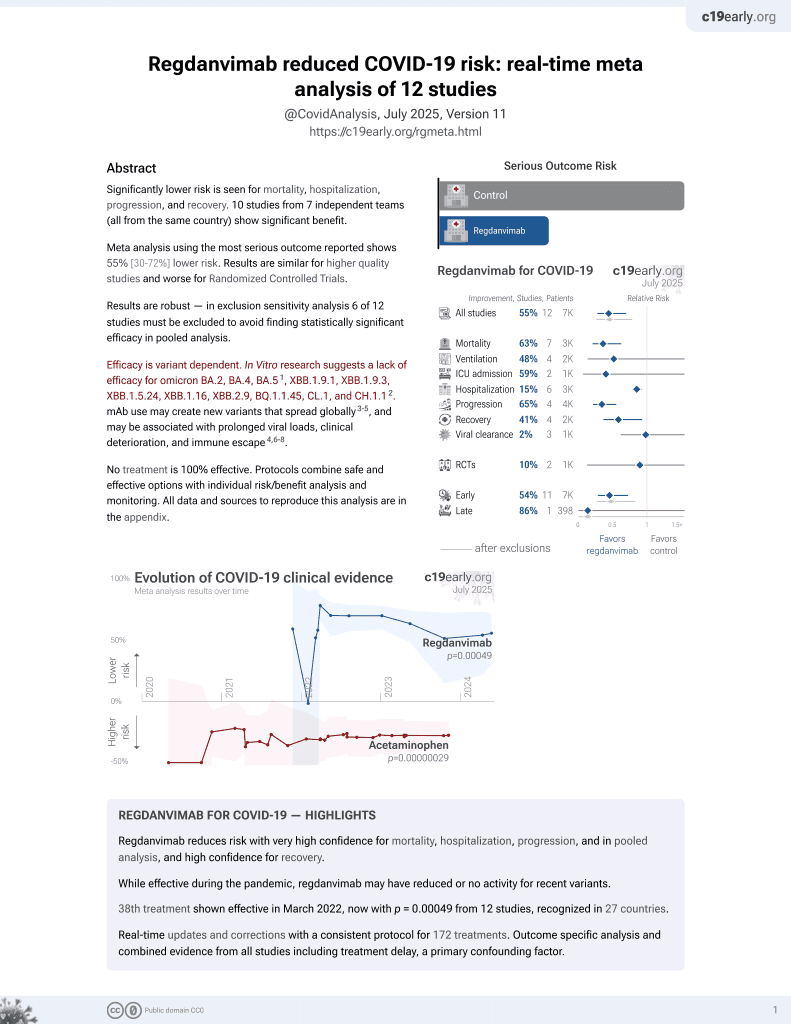
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Phase 1 RCT of 23 healthy adults, showing nebulized IN-006, a reformulation of regdanvimab, was well-tolerated, with transient mild adverse events, and achieved high nasal fluid concentrations that were orders of magnitude above typical antiviral mAb IC50 values within minutes of dosing.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
Moench et al., 21 Aug 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Australia, peer-reviewed, 17 authors, study period 22 September, 2021 - 29 December, 2021, trial ACTRN12621001235897.
Contact: moench@inhalon.com, lai@inhalon.com.
A randomized, double-blind, Phase 1, single- and multiple-dose placebo-controlled study of the safety and pharmacokinetics of IN-006, an inhaled antibody treatment for COVID-19 in healthy volunteers
eBioMedicine, doi:10.1016/j.ebiom.2025.105582
Background Although COVID-19 is predominantly a respiratory tract infection, current antibody treatments are administered by systemic dosing. We hypothesize that inhaled delivery of a monoclonal antibody may be a more effective and convenient route. We investigated the safety, tolerability, and pharmacokinetics of IN-006, a reformulation of regdanvimab for nebulized delivery by a handheld nebulizer. Methods A Phase 1 study was conducted in healthy volunteers aged 18-55 a Phase 1 unit in Melbourne, Australia (ACTRN12621001235897). Study staff and participants were blinded to treatment assignment, except for pharmacy staff preparing the study drug. The ratio of active:placebo randomization to each cohort was set at 3:1. The primary outcomes were safety and tolerability. Exploratory outcomes were pharmacokinetics of IN-006 in nasal fluid and serum. Findings Twenty-three participants were enrolled and randomized across two single dose and one multiple dose cohorts (30 mg or 90 mg single nebulized dose, or seven daily 90 mg doses). There were no serious adverse events. All enrolled participants completed the study without treatment interruption or discontinuation. All treatmentemergent adverse events were transient, non-dose dependent, and graded mild to moderate in severity. Nebulization was well-tolerated and completed in an average of 6 min. Geometric mean nasal fluid concentrations of IN-006 in the multiple dose cohort were 739.8 μg/mL at 30 min after dosing and 1.2 μg/mL at 22 h. Geometric mean serum levels in the multiple dose cohort peaked at 0.51 μg/mL 3 days after the final dose. Interpretation IN-006 was well-tolerated and achieved concentrations in the respiratory tract orders of magnitude above the IC 50 range typical of antiviral mAbs. These data support further development of nebulized delivery of antiviral mAbs for respiratory infectious disease.
The academic authors and the authors who are employees of the sponsor vouch for the accuracy and completeness of the data, the statistical analysis, and the fidelity of the study to the protocol.
Data sharing statement Deidentified data from this study will be made available from the corresponding author upon reasonable request. Reagents related to this study may be available upon reasonable request submitted to the corresponding author, pending availability. Some reagents associated with this study, including IN-006, are no longer clinically available and therefore cannot be shared.
Declaration of interests TM, LB, BF, MH, MB, MM, ZR, JW, FF, and JH are employees of Inhalon Biopharma/Mucommune and may hold shares in Inhalon Biopharma, Inc. HK, YP, CK, JMC, and SYL are employees of Celltrion, Inc, which received funding from the government of Korea to advance Regdanvimab, which was used to cover salary for their work on this project. JL received partial salary support for his work on this project. SKL is founder of Mucommune, LLC and currently serves as its interim CEO. SKL is also founder of Inhalon Biopharma, Inc, and currently serves as its CSO as well as on its Board of Director and Scientific Advisory Board. S.K.L has equity interests in both Mucommune and Inhalon Biopharma; S.K.L's relationships with Mucommune and Inhalon are subject to certain restrictions under University policy. The terms of these arrangements are managed by UNC-CH in accordance with its conflict..
References
Bodier-Montagutelli, Respaud, Perret, Protein stability during nebulization: mind the collection step, Eur J Pharm Biopharm
Bodier-Montagutelli, Vecellio, Respaud, Heuzé-Vourc H N, Designing inhaled protein therapeutics for topical lung delivery: what are the next steps? Expet, Opin Drug Deliv
Chen, Bobrovitz, Premji, Koopmans, Fisman et al., SARS-CoV-2 shedding dynamics across the respiratory tract, sex, and disease severity for adult and pediatric COVID-19, Elife
Cruz-Teran, Tiruthani, Mcsweeney, Ma, Pickles et al., Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy, Adv Drug Deliv Rev
Dall'acqua, Kiener, Wu, Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn), J Biol Chem
Darquenne, Aerosol deposition in health and disease, J Aerosol Med Pulm Drug Deliv
Emma, Caponnetto, Cibella, Short and long term repeatability of saccharin transit time in current, former, and never smokers, Front Physiol
Han, Czajkowski, Rosas, Safety and efficacy of CR6261 in an influenza A H1N1 healthy human challenge model, Clin Infect Dis
Hart, Cook, Amirhosseini, Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys, J Allergy Clin Immunol
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Jia, Look, Shi, ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia, J Virol
Lai, Mcsweeney, Pickles, Learning from past failures: challenges with monoclonal antibody therapies for COVID-19, J Control Release
Lee, Nakayama, Wu, ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs, Nat Commun
Lim, Cheong, Oh, Modeling the early temporal dynamics of viral load in respiratory tract specimens of COVID-19 patients in Incheon, the Republic of Korea, Int J Infect Dis
Liu, Iketani, Guo, Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2, Nature
Loira-Pastoriza, Todoroff, Vanbever, Delivery strategies for sustained drug release in the lungs, Adv Drug Deliv Rev
Mayor, Thibert, Huille, Respaud, Audat et al., Inhaled antibodies: formulations require specific development to overcome instability due to nebulization, Drug Deliv Transl Res
Mcsweeney, Stewart, Richardson, Stable nebulization and muco-trapping properties of Regdanvimab/IN-006 supports its development as a potent, dose-saving inhaled therapy for COVID-19, bioRxiv, doi:10.1101/2022.02.27.482162
Mcsweeney, Stewart, Richardson, Stable nebulization and muco-trapping properties of regdanvimab/IN-006 support its development as a potent, dose-saving inhaled therapy for COVID-19, Bioeng Transl Med
Nagy, Leach, King, Guttendorf, Safety, pharmacokinetics, and immunogenicity of obiltoxaximab after intramuscular administration to healthy humans, Clin Pharmacol Drug Dev
Pinto, Rai, Brown, Ultrastructural insight into SARS-CoV-2 entry and budding in human airway epithelium, Nat Commun
Respaud, Vecellio, Diot, Heuze-Vourc'h N, Nebulization as a delivery method for mAbs in respiratory diseases, Expet Opin Drug Deliv
Rodrigues, Freire, Uzeloto, Particularities and clinical applicability of saccharin transit time test, Int Arch Otorhinolaryngol
Sims, Burkett, Yount, Pickles, SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium, Virus Res
Suri, The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis, BioDrugs
Syed, Regdanvimab: first approval, Drugs
Throsby, Van Den Brink, Jongeneelen, Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells, PLoS One
Van Der Straten, Van Gils, De Taeye, De Bree, Optimization of anti-SARS-CoV-2 neutralizing antibody therapies: roadmap to improve clinical effectiveness and implementation, Front Med Technol
Wu, Pfarr, Johnson, Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract, J Mol Biol
Zhang, Bukreyev, Thompson, Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium, J Virol
Zhang, Peeples, Boucher, Collins, Pickles, Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology, J Virol
Zhu, Mclellan, Kallewaard, A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants, Sci Transl Med
DOI record:
{
"DOI": "10.1016/j.ebiom.2025.105582",
"ISSN": [
"2352-3964"
],
"URL": "http://dx.doi.org/10.1016/j.ebiom.2025.105582",
"alternative-id": [
"S235239642500026X"
],
"article-number": "105582",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A randomized, double-blind, Phase 1, single- and multiple-dose placebo-controlled study of the safety and pharmacokinetics of IN-006, an inhaled antibody treatment for COVID-19 in healthy volunteers"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eBioMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ebiom.2025.105582"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Published by Elsevier B.V."
}
],
"author": [
{
"affiliation": [],
"family": "Moench",
"given": "Thomas R.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Botta",
"given": "Lakshmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Farrer",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lickliter",
"given": "Jason D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Hyunah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Yoona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Cheolmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoke",
"given": "Marshall",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brennan",
"given": "Miles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McSweeney",
"given": "Morgan D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Zachary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Whelan",
"given": "John B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cho",
"given": "Jong Moon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Soo Young",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faurot",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hutchins",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lai",
"given": "Samuel K.",
"sequence": "additional"
}
],
"container-title": "eBioMedicine",
"container-title-short": "eBioMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
2,
8
]
],
"date-time": "2025-02-08T16:39:03Z",
"timestamp": 1739032743000
},
"deposited": {
"date-parts": [
[
2025,
2,
8
]
],
"date-time": "2025-02-08T16:39:13Z",
"timestamp": 1739032753000
},
"indexed": {
"date-parts": [
[
2025,
2,
8
]
],
"date-time": "2025-02-08T17:10:28Z",
"timestamp": 1739034628117,
"version": "3.37.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T00:00:00Z",
"timestamp": 1740787200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
1
]
],
"date-time": "2025-03-01T00:00:00Z",
"timestamp": 1740787200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
18
]
],
"date-time": "2025-01-18T00:00:00Z",
"timestamp": 1737158400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642500026X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S235239642500026X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105582",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
3
]
]
},
"published-print": {
"date-parts": [
[
2025,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41467-022-29255-y",
"article-title": "Ultrastructural insight into SARS-CoV-2 entry and budding in human airway epithelium",
"author": "Pinto",
"doi-asserted-by": "crossref",
"first-page": "1609",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.ebiom.2025.105582_bib1",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-19145-6",
"article-title": "ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "5453",
"issue": "1",
"journal-title": "Nat Commun",
"key": "10.1016/j.ebiom.2025.105582_bib2",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1128/JVI.79.23.14614-14621.2005",
"article-title": "ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia",
"author": "Jia",
"doi-asserted-by": "crossref",
"first-page": "14614",
"issue": "23",
"journal-title": "J Virol",
"key": "10.1016/j.ebiom.2025.105582_bib3",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1128/JVI.76.11.5654-5666.2002",
"article-title": "Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "5654",
"issue": "11",
"journal-title": "J Virol",
"key": "10.1016/j.ebiom.2025.105582_bib4",
"volume": "76",
"year": "2002"
},
{
"DOI": "10.1016/j.virusres.2007.03.013",
"article-title": "SARS-CoV replication and pathogenesis in an in vitro model of the human conducting airway epithelium",
"author": "Sims",
"doi-asserted-by": "crossref",
"first-page": "33",
"issue": "1",
"journal-title": "Virus Res",
"key": "10.1016/j.ebiom.2025.105582_bib5",
"volume": "133",
"year": "2008"
},
{
"DOI": "10.1128/JVI.79.2.1113-1124.2005",
"article-title": "Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1113",
"issue": "2",
"journal-title": "J Virol",
"key": "10.1016/j.ebiom.2025.105582_bib6",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"article-title": "SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "429",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.ebiom.2025.105582_bib7",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1016/j.jconrel.2020.11.057",
"article-title": "Learning from past failures: challenges with monoclonal antibody therapies for COVID-19",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "87",
"journal-title": "J Control Release",
"key": "10.1016/j.ebiom.2025.105582_bib8",
"volume": "329",
"year": "2021"
},
{
"DOI": "10.1016/j.addr.2020.12.004",
"article-title": "Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy",
"author": "Cruz-Teran",
"doi-asserted-by": "crossref",
"first-page": "100",
"journal-title": "Adv Drug Deliv Rev",
"key": "10.1016/j.ebiom.2025.105582_bib9",
"volume": "169",
"year": "2021"
},
{
"DOI": "10.1067/mai.2001.116576",
"article-title": "Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys",
"author": "Hart",
"doi-asserted-by": "crossref",
"first-page": "250",
"issue": "2",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.ebiom.2025.105582_bib10",
"volume": "108",
"year": "2001"
},
{
"DOI": "10.1074/jbc.M604292200",
"article-title": "Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn)",
"author": "Dall'Acqua",
"doi-asserted-by": "crossref",
"first-page": "23514",
"issue": "33",
"journal-title": "J Biol Chem",
"key": "10.1016/j.ebiom.2025.105582_bib11",
"volume": "281",
"year": "2006"
},
{
"DOI": "10.1093/cid/ciaa1725",
"article-title": "Safety and efficacy of CR6261 in an influenza A H1N1 healthy human challenge model",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "e4260",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ebiom.2025.105582_bib12",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.3389/fmedt.2022.867982",
"article-title": "Optimization of anti-SARS-CoV-2 neutralizing antibody therapies: roadmap to improve clinical effectiveness and implementation",
"author": "van der Straten",
"doi-asserted-by": "crossref",
"journal-title": "Front Med Technol",
"key": "10.1016/j.ebiom.2025.105582_bib13",
"volume": "4",
"year": "2022"
},
{
"DOI": "10.1089/jamp.2011.0916",
"article-title": "Aerosol deposition in health and disease",
"author": "Darquenne",
"doi-asserted-by": "crossref",
"first-page": "140",
"issue": "3",
"journal-title": "J Aerosol Med Pulm Drug Deliv",
"key": "10.1016/j.ebiom.2025.105582_bib14",
"volume": "25",
"year": "2012"
},
{
"article-title": "Stable nebulization and muco-trapping properties of regdanvimab/IN-006 support its development as a potent, dose-saving inhaled therapy for COVID-19",
"author": "McSweeney",
"journal-title": "Bioeng Transl Med",
"key": "10.1016/j.ebiom.2025.105582_bib15",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.3389/fphys.2020.01109",
"article-title": "Short and long term repeatability of saccharin transit time in current, former, and never smokers",
"author": "Emma",
"doi-asserted-by": "crossref",
"first-page": "1109",
"journal-title": "Front Physiol",
"key": "10.1016/j.ebiom.2025.105582_bib16",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1055/s-0038-1676116",
"article-title": "Particularities and clinical applicability of saccharin transit time test",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"first-page": "229",
"issue": "2",
"journal-title": "Int Arch Otorhinolaryngol",
"key": "10.1016/j.ebiom.2025.105582_bib17",
"volume": "23",
"year": "2019"
},
{
"DOI": "10.1007/s40265-021-01626-7",
"article-title": "Regdanvimab: first approval",
"author": "Syed",
"doi-asserted-by": "crossref",
"first-page": "2133",
"issue": "18",
"journal-title": "Drugs",
"key": "10.1016/j.ebiom.2025.105582_bib18",
"volume": "81",
"year": "2021"
},
{
"DOI": "10.1016/j.jmb.2007.02.024",
"article-title": "Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "652",
"issue": "3",
"journal-title": "J Mol Biol",
"key": "10.1016/j.ebiom.2025.105582_bib19",
"volume": "368",
"year": "2007"
},
{
"DOI": "10.1126/scitranslmed.aaj1928",
"article-title": "A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants",
"author": "Zhu",
"doi-asserted-by": "crossref",
"issue": "388",
"journal-title": "Sci Transl Med",
"key": "10.1016/j.ebiom.2025.105582_bib20",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1517/17425247.2015.999039",
"article-title": "Nebulization as a delivery method for mAbs in respiratory diseases",
"author": "Respaud",
"doi-asserted-by": "crossref",
"first-page": "1027",
"issue": "6",
"journal-title": "Expet Opin Drug Deliv",
"key": "10.1016/j.ebiom.2025.105582_bib21",
"volume": "12",
"year": "2015"
},
{
"DOI": "10.1007/s13346-021-00967-w",
"article-title": "Inhaled antibodies: formulations require specific development to overcome instability due to nebulization",
"author": "Mayor",
"doi-asserted-by": "crossref",
"first-page": "1625",
"issue": "4",
"journal-title": "Drug Deliv Transl Res",
"key": "10.1016/j.ebiom.2025.105582_bib22",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.ejpb.2020.04.006",
"article-title": "Protein stability during nebulization: mind the collection step",
"author": "Bodier-Montagutelli",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Eur J Pharm Biopharm",
"key": "10.1016/j.ebiom.2025.105582_bib23",
"volume": "152",
"year": "2020"
},
{
"DOI": "10.1080/17425247.2018.1503251",
"article-title": "Designing inhaled protein therapeutics for topical lung delivery: what are the next steps?",
"author": "Bodier-Montagutelli",
"doi-asserted-by": "crossref",
"first-page": "729",
"issue": "8",
"journal-title": "Expet Opin Drug Deliv",
"key": "10.1016/j.ebiom.2025.105582_bib24",
"volume": "15",
"year": "2018"
},
{
"DOI": "10.1016/j.addr.2014.05.017",
"article-title": "Delivery strategies for sustained drug release in the lungs",
"author": "Loira-Pastoriza",
"doi-asserted-by": "crossref",
"first-page": "81",
"journal-title": "Adv Drug Deliv Rev",
"key": "10.1016/j.ebiom.2025.105582_bib25",
"volume": "75",
"year": "2014"
},
{
"DOI": "10.2165/00063030-200519030-00001",
"article-title": "The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis",
"author": "Suri",
"doi-asserted-by": "crossref",
"first-page": "135",
"issue": "3",
"journal-title": "BioDrugs",
"key": "10.1016/j.ebiom.2025.105582_bib26",
"volume": "19",
"year": "2005"
},
{
"DOI": "10.1038/s41586-021-04388-0",
"article-title": "Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "676",
"issue": "7898",
"journal-title": "Nature",
"key": "10.1016/j.ebiom.2025.105582_bib27",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1002/cpdd.410",
"article-title": "Safety, pharmacokinetics, and immunogenicity of obiltoxaximab after intramuscular administration to healthy humans",
"author": "Nagy",
"doi-asserted-by": "crossref",
"first-page": "652",
"issue": "6",
"journal-title": "Clin Pharmacol Drug Dev",
"key": "10.1016/j.ebiom.2025.105582_bib28",
"volume": "7",
"year": "2018"
},
{
"article-title": "Stable nebulization and muco-trapping properties of Regdanvimab/IN-006 supports its development as a potent, dose-saving inhaled therapy for COVID-19",
"author": "McSweeney",
"journal-title": "bioRxiv",
"key": "10.1016/j.ebiom.2025.105582_bib29",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0003942",
"article-title": "Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells",
"author": "Throsby",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "PLoS One",
"key": "10.1016/j.ebiom.2025.105582_bib30",
"volume": "3",
"year": "2008"
},
{
"DOI": "10.7554/eLife.70458",
"article-title": "SARS-CoV-2 shedding dynamics across the respiratory tract, sex, and disease severity for adult and pediatric COVID-19",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Elife",
"key": "10.1016/j.ebiom.2025.105582_bib31",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.05.062",
"article-title": "Modeling the early temporal dynamics of viral load in respiratory tract specimens of COVID-19 patients in Incheon, the Republic of Korea",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "428",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.ebiom.2025.105582_bib32",
"volume": "108",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S235239642500026X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "A randomized, double-blind, Phase 1, single- and multiple-dose placebo-controlled study of the safety and pharmacokinetics of IN-006, an inhaled antibody treatment for COVID-19 in healthy volunteers",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "113"
}