COVID-19 Prophylactic Effect of Bromhexine Hydrochloride
et al., Preprints.org, doi:10.20944/preprints202410.1998.v1, Oct 2024
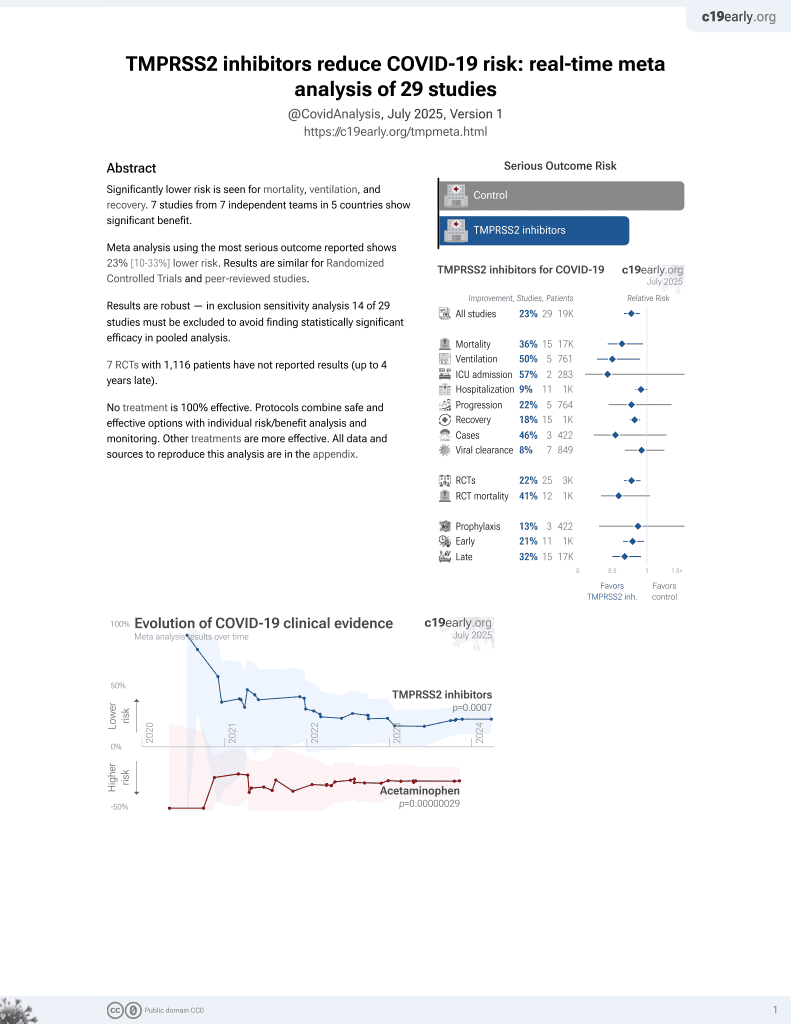
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
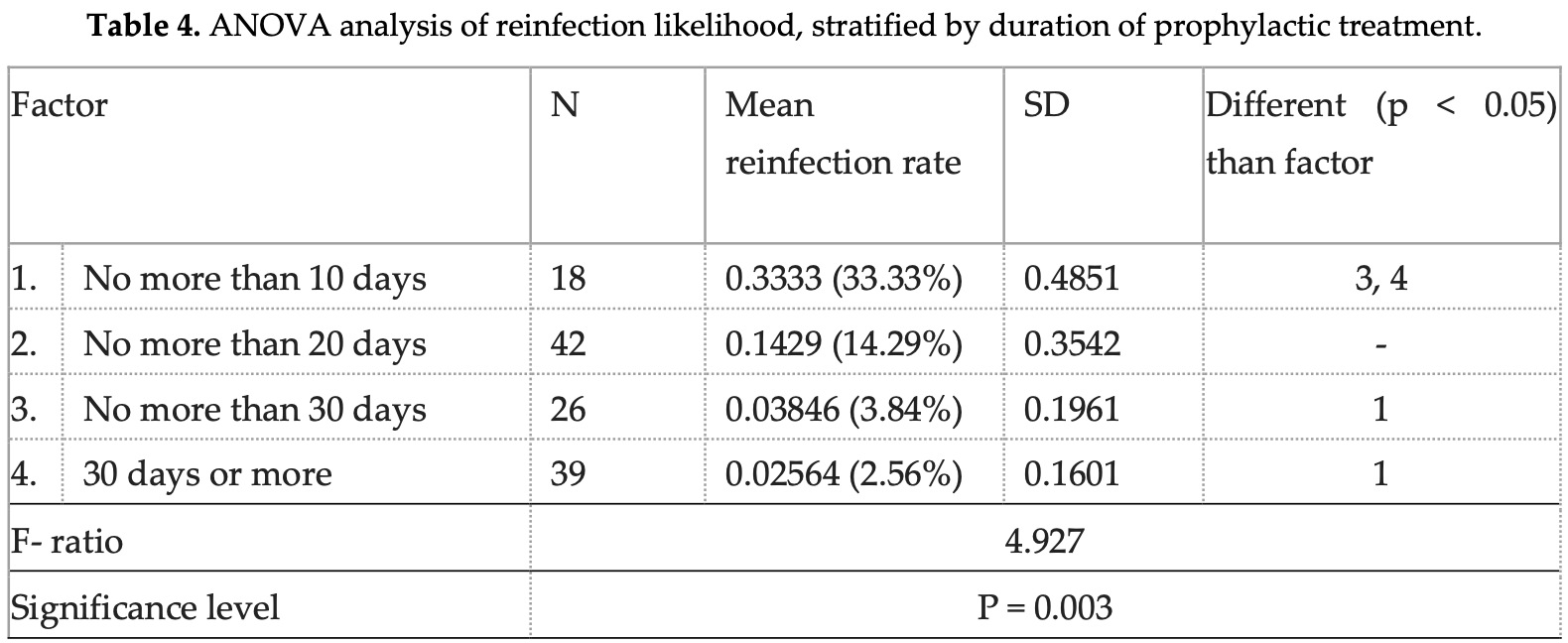
Retrospective 125 outpatients showing reduced COVID-19 infection rates with prophylactic bromhexine hydrochloride (BRH) use during 2021-2022 COVID waves in Bulgaria. Prior to BRH prophylaxis, 62% of participants reported confirmed COVID-19 diagnoses, which decreased to 11% after BRH initiation (p<0.0001). The protective effect was more pronounced with longer duration of prophylactic use - patients taking BRH for >30 days had significantly lower reinfection rates (3%) compared to those taking it for ≤10 days (33%). Reliance on patient-reported outcomes, potential recall bias, lack of control for social contact levels, and lack of a concurrent control group limit conclusions.
Study covers TMPRSS2 inhibitors and bromhexine.
Mitev et al., 25 Oct 2024, retrospective, Bulgaria, preprint, 5 authors.
COVID-19 Prophylactic Effect of Bromhexine Hydrochloride
doi:10.20944/preprints202410.1998.v1
Despite the enormous efforts and funds spent to find an effective treatment for COVID-19, the results have been disappointing. In previous publications, we have demonstrated the remarkable effect of high-dose colchicine in inhibiting the cytokine storm and preventing multiorgan damage and death. However, this treatment is beneficial only after virus entry into the cell. The question of prophylaxis and entry prohibition should also be explored. We now demonstrate the prophylactic effect of bromhexine hydrochloride (BRH), an over-the-counter, noninvasive, effective, well-tolerated medicine, with proven safety, affordable, and inexpensive on 125 men and women. The effect of BRH is best when given continuously for prophylaxis during peaks in contagion in the wave of COVID-19. Then the probability of infection drops sharply, and if a disease does occur, it proceeds mildly. BRH is also effective when given by inhalation for postexposure prophylaxis. When COVID-19 manifests itself clinically, the efficacy of BRH drops sharply because the virus is already in the cell. However, BRH inhalations are useful because they limit the spread of the virus and have an anti-inflammatory and possibly antiviral effect.
Supplementary Materials: The following supporting information can be downloaded at: Preprints.org.
References
Ansarin, Tolouian, Ardalan, Taghizadieh, Varshochi et al., Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial, BioImpacts
Atyeo, Perez, Matuck, Byrd, Warner, The Mouth as a Site of SARS-CoV-2 Infection, Curr Oral Health Rep
Bahadoram, Keikhaei, Bahadoram, Mahmoudian-Sani, Hassanzadeh et al., Bromhexine is a potential drug for COVID-19; From hypothesis to clinical trials, Vopr Virusol
Boechat, Chora, Morais, Delgado, The immune response to SARS-CoV-2 and COVID-19 immunopathology -Current perspectives, Pulmonology
Bulanov, Yonkov, Arabadzhieva, Mitev, Successful Treatment With High-Dose Colchicine of a 101-Year-Old Patient Diagnosed With COVID-19 After an Emergency Cholecystectomy, Cureus
Böttcher, Matrosovich, Beyerle, Klenk, Garten et al., Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium, J Virol
Chupp, Spichler-Moffarah, Søgaard, Esserman, Dziura et al., Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste
Cuerdo, Ogbac, Tamayo, Effect of Bromhexine among COVID-19 Patients -A Meta-Anaylsis, ERJ Open Res
Depfenhart, De Villiers, Lemperle, Meyer, Di Somma, Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?, Intern Emerg Med
Freeman, Swartz, Targeting the NLRP3 Inflammasome in Severe COVID-19, Front. Immunol
Fu, Wang, Yuan, Chen, Ao et al., Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and metaanalysis, Journal of Infection
Fu, Zheng, Zhou, Tang, Chen et al., Re-recognizing bromhexine hydrochloride: pharmaceutical properties and its possible role in treating pediatric COVID-19, Eur J Clin Pharmacol
Fung, Yuen, Ye, Chan, Jin, A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses, Emerging Microbes Infections
Garten, Braden, Arendt, Peitsch, Baron et al., Influenza virus activating host proteases: Identification, localization and inhibitors as potential therapeutics, Eur J Cell Biol
Ghayour, Nazari, Keramat, Shahbazi, Eslami-Ghayour, Evaluation of the efficacy of Nacetylcysteine and bromhexine compared with standard care in preventing hospitalization of outpatients with COVID-19: a double blind randomized clinical trial, Rev Clín Esp
Gheblawi, Wang, Viveiros, Nguyen, Zhong et al., Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the reninangiotensin system: Celebrating the 20th anniversary of the discovery of ACE2, Circ. Res
Han, Mallampalli, The role of surfactant in lung disease and host defense against pulmonary infections, Annals of the American Thoracic Society
He, Lau, Wu, Deng, Wang et al., Temporal dynamics in viral shedding and transmissibility of COVID-19, Nature medicine
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Huynh, Wang, Luan, In silico exploration of the molecular mechanism of clinically oriented drugs for possibly inhibiting SARS-CoV-2's main protease, J. Phys. Chem. Lett
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection, J Vvirol
Jackson, Farzan, Chen, Choe, Mechanisms of SARS-CoV-2 entry into cells, Nat Rev Mol Cell Biol
Khan, Mubariz, Khlidj, Nasirk, Ramadan et al., Safety and Efficacy of Camostat Mesylate for Covid-19: a systematic review and Meta-analysis of Randomized controlled trials, BMC Infect Dis
Kinoshita, Shinoda, Nishizaki, Shiraki, Hirai et al., A multicenter, double-blind, randomized, parallel-group, placebocontrolled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study), BMC Med
Lee, Lee, Kim, Ko, Jee et al., TMPRSS2 and RNA-dependent RNA polymerase are effective targets of therapeutic intervention for treatment of COVID-19 caused by SARS-CoV-2 variants (B. 1.1. 7 and B. 1.351), Microbiol Spectr
Lilov, Palaveev, Mitev, High Doses of Colchicine Act As "Silver Bullets" Against Severe COVID-19, Cureus
Limburg, Harbig, Bestle, Stein, Moulton et al., TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes, J Virol
Lucas, Heinlein, Kim, Hernandez, Malik et al., The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microen-vironment and promotes prostate cancer metastasis, Cancer Discovery
Maggio, Corsini, Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection, Pharmacol. Res
Markus, Gottfried, Markus, Marina, Dario et al., A SARS-CoV-2 prophylactic and treatment; A counter argument against the sole use of chloroquine, Am J Biomed Sci
Mehta, Mcauley, Brown, Sanchez, Tattersal et al., COVID-19: consider cytokine storm syndromes and immunosuppression, The Lancet
Mikhaylov, Lyubimtseva, Vakhrushev, Stepanov, Lebedev et al., Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study, Interdiscip Perspect Infect Dis
Mitev, Colchicine-The Divine Medicine against COVID-19, J. Pers. Med
Mitev, Comparison of treatment of COVID-19 with inhaled bromhexine, higher doses of colchicine and hymecromone with WHO-recommended paxlovid, molnupiravir, remdesivir, anti-IL-6 receptor antibodies and baricitinib, Pharmacia
Mitev, Mondeshki, Marinov, Bilukov, Colchicine, bromhexine, and hymecromone as part of COVID19 treatment -cold, warm, hot
Mitev, What is the lowest lethal dose of colchicine?, Biotechnol. Biotechnol. Equip
Mondeshki, Bilyukov, Mitev, Effect of an Accidental Colchicine Overdose in a COVID-19 Inpatient With Bilateral Pneumonia and Pericardial Effusion, Cureus
Mondeshki, Bilyukov, Tomov, Mihaylov, Mitev, Complete, rapid resolution of severe bilateral pneumonia and acute respiratory distress syndrome in a COVID-19 patient: role for a unique therapeutic combination of inhalations with bromhexine, higher doses of colchicine, and hymecromone, Cureus
Mondeshki, Mitev, High-Dose Colchicine: Key Factor in the Treatment of Morbidly Obese COVID-19 Patients, Cureus
Morgenstern, Paxlovid evidence: Still very little reason to prescribe, First
Méndez, Antón Sanz, Cárdenas García, Bravo Malo, Torres Martínez et al., Efficacy of bromhexine versus standard of care in reducing viral load in patients with mild-to-moderate COVID-19 disease attended in primary care: A randomized open-label trial, J Clin Med
Ni, Bin, Li-Li, The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies, Cytokine Growth Factor Rev
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat Commun
Plomer, De Zeeuw, More than expectorant: new scientific data on ambroxol in the context of the treatment of bronchopulmonary diseases, MMW-Fortschritte der Medizin
Sakai, Ami, Nakajima, Nakajima, Kitazawa et al., TMPRSS2 independency for haemagglutinin cleavage in vivo differentiates influenza B virus from influenza A virus, Sci Rep
Sax, The Rise and Fall of Paxlovid-HIV and ID Observations, NEJM Journal Watch
Shen, Mao, Wu, Tanaka, Zhang, TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections, Biochimie
Shen, Mao, Wu, Tanaka, Zhang, TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections, Biochimie
Shirato, Kawase, Matsuyama, Wild-type human coronaviruses prefer cell-surface TMPRSS2 to endosomal cathepsins for cell entry, Virology
Tiholov, Lilov, Georgieva, Palaveev, Tashkov et al., Effect of increasing doses of colchicine on thetreatment of 333 COVID-19 inpatients, Immun Inflamm Dis
Tobback, Degroote, Buysse, Delesie, Van Dooren et al., Efficacy and safety of camostat mesylate in early COVID-19 disease in an ambulatory setting: a randomized placebo-controlled phase II trial, Int J Infect Dis
Tolouian, Moradi, Mulla, Ziaie, Haghighi et al., Bromhexine for post-exposure COVID-19 prophylaxis: A randomized, double-blind, placebo-controlled trial, Jundishapur J. Microbiol
Tolouian, Mulla, Controversy with bromhexine in COVID-19; where we stand, Immunopathologia Persa
Tolouian, Mulla, Jamaati, Babamahmoodi, Marjani et al., Effect of bromhexine in hospitalized patients with COVID-19, JIM
Tsampasian, Elghazaly, Chattopadhyay, Risk Factors Associated With Post-COVID-19 Condition: A Systematic Review and Meta-analysis, JAMA Intern Med, doi:10.1001/jamainternmed.2023.0750
V'kovski, Kratzel, Steiner, Stalder, Thiel, Coronavirus biology and replication: implications for SARS-CoV-2, Nat Rev Microbiol
Wettstein, Kirchhoff, Münch, The Transmembrane Protease TMPRSS2 as a Therapeutic Target for COVID-19 Treatment, Int. J. Mol. Sci
Zhang, Penninger, Li, Zhong, Slutsky, Angio-tensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target, Intensive Care Med
Zhang, Wu, Li, Zhao, Wang, Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality, Int. J. Antimicrob. Agents
Zhao, Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies, Int. J. Antimicrob. Agents
Zhao, Di, Xu, The NLRP3 inflammasome and COVID-19: Activation, pathogenesis and therapeutic strategies, Cytokine Growth Factor Rev
Zhao, Yang, Yang, Zhang, Huang et al., Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development, Signal Transduct Target Ther
Zhou, Vedantham, Lu, Agudelo, Carrion et al., Protease inhibitors targeting coronavirus and filovirus entry, Antiviral Res