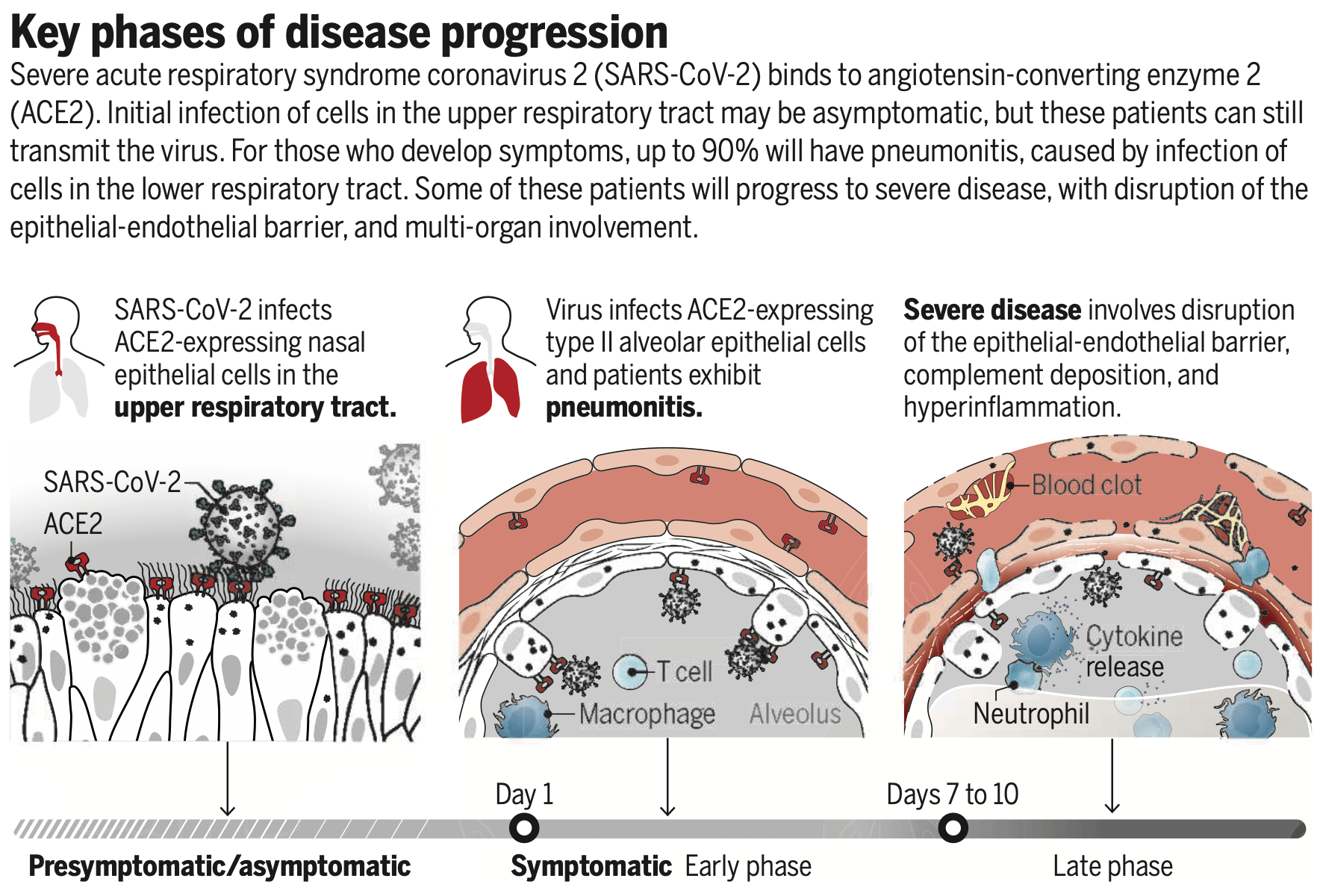
Review discussing how the viral receptor ACE2 regulates SARS-CoV-2 cell entry, tissue tropism, and COVID-19 disease progression. The presence of a furin cleavage site in the SARS-CoV-2 spike protein expands cell tropism compared to SARS-CoV. Expression of ACE2 and the TMPRSS2 protease is highest in the upper respiratory tract, enabling efficient transmission of SARS-CoV-2 before symptom onset. Severe COVID-19 is characterized by breakdown of the lung epithelial-endothelial barrier and may be driven by persistent viral replication and/or immune-mediated damage.
Matheson et al., 31 Jul 2020, peer-reviewed, 2 authors.
Contact: pjl30@cam.ac.uk, njm25@cam.ac.uk.
Abstract: INSIGHTS | P E R S P E C T I V E S
VIEWPOINT: COVID-19
How does SARS-CoV-2 cause COVID-19?
The viral receptor on human cells plays a critical role in disease progression
By Nicholas J. Matheson1,2 and Paul J. Lehner1
V
1
Department of Medicine, Cambridge Institute for
Therapeutic Immunology and Infectious Disease (CITIID),
University of Cambridge, Cambridge, UK.2NHS Blood
and Transplant, Cambridge, UK. Email: pjl30@cam.ac.uk;
njm25@cam.ac.uk
510
without targeting ACE2, so other factors
must also be important.
As a respiratory virus, SARS-CoV-2 must
initially enter cells lining the respiratory
tract. Single-cell sequencing and RNA in situ
mapping of the human respiratory tract show
ACE2 and TMPRSS2 expression to be highest
in ciliated nasal epithelial cells, with lesser
amounts in ciliated bronchial epithelial cells
and type II alveolar epithelial cells (6). This
translates to greater permissivity of upper
versus lower respiratory tract epithelial cells
for SARS-CoV-2 infection in vitro and fits
disease pathology: Upper respiratory tract
symptoms are common early in disease, with
nasopharyngeal and throat swabs positive for
SARS-CoV-2 at clinical presentation (7). In
contrast to SARS-CoV, infectivity of patients
with SARS-CoV-2 peaks before symptom onset (8). Indeed, presymptomatic transmission
makes SARS-CoV-2 impossible to contain
through case isolation alone and is a key
driver of the pandemic (8). This alteration in
the pattern of disease may relate to the acquisition of the furin cleavage site in spike or
increased binding affinity for ACE2 in SARSCoV-2, compared with SARS-CoV (9).
If the main role of ACE2 is to cleave angiotensin II, it is unclear why expression in
lung tissue is more prominent in epithelial
than in endothelial cells. Furthermore, the
Human Cell Atlas highlights ACE2 expression in intestinal enterocytes, rather than in
the lungs. This distribution may reflect nonenzymatic roles of ACE2, such as chaperoning amino acid transporters. Indeed, SARSCoV-2 infection of the gastrointestinal (GI)
tract is common, with viral RNA detectable
in stool in up to 30% of COVID-19 patients.
This likely contributes to the frequency of GI
symptoms. Conversely, whereas fecal-oral
transmission of coronaviruses is thought to
be prominent among bats, it appears to be
a minor transmission route for SARS-CoV-2
in humans, perhaps because colonic fluid
inactivates the virus. Whether extrapulmonary ACE2 expression and concomitant
viral infection account for other clinical
manifestations of SARS-CoV-2 is unclear.
The association between SARS-CoV-2 infection and anosmia (loss of smell) may reflect
ACE2 and TMPRSS2 expression in sustentacular cells, which maintain the integrity
of olfactory sensory neurons. Olfactory
epithelial cells also express NRP1 and could
provide a direct route to the brain (4).
sciencemag.org SCIENCE
31 JULY 2020 • VOL 369 ISSUE 6503
Published by AAAS
iruses enter cells and initiate infection by binding to their cognate cell
surface receptors. The expression and
distribution of viral entry receptors
therefore regulates their tropism, determining the tissues that are infected
and thus disease pathogenesis. Severe acute
respiratory syndrome coronavirus 2 (SARSCoV-2) is the third human coronavirus
known to co-opt the peptidase angiotensinconverting enzyme 2 (ACE2) for cell entry
(1). The interaction between SARS-CoV-2 and
ACE2 is critical to determining both..
DOI record:
{
"DOI": "10.1126/science.abc6156",
"ISSN": [
"0036-8075",
"1095-9203"
],
"URL": "http://dx.doi.org/10.1126/science.abc6156",
"abstract": "<jats:p>The viral receptor on human cells plays a critical role in disease progression</jats:p>",
"alternative-id": [
"10.1126/science.abc6156"
],
"author": [
{
"affiliation": [
{
"name": "Department of Medicine, Cambridge Institute for Therapeutic Immunology and Infectious Disease (CITIID), University of Cambridge, Cambridge, UK."
},
{
"name": "NHS Blood and Transplant, Cambridge, UK."
}
],
"family": "Matheson",
"given": "Nicholas J.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine, Cambridge Institute for Therapeutic Immunology and Infectious Disease (CITIID), University of Cambridge, Cambridge, UK."
}
],
"family": "Lehner",
"given": "Paul J.",
"sequence": "additional"
}
],
"container-title": "Science",
"container-title-short": "Science",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
30
]
],
"date-time": "2020-07-30T19:06:39Z",
"timestamp": 1596135999000
},
"deposited": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T18:32:08Z",
"timestamp": 1705343528000
},
"indexed": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T13:59:01Z",
"timestamp": 1718632741323
},
"is-referenced-by-count": 153,
"issue": "6503",
"issued": {
"date-parts": [
[
2020,
7,
31
]
]
},
"journal-issue": {
"issue": "6503",
"published-print": {
"date-parts": [
[
2020,
7,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.sciencemag.org/about/science-licenses-journal-article-reuse",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
31
]
],
"date-time": "2020-07-31T00:00:00Z",
"timestamp": 1596153600000
}
}
],
"link": [
{
"URL": "https://www.science.org/doi/pdf/10.1126/science.abc6156",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "221",
"original-title": [],
"page": "510-511",
"prefix": "10.1126",
"published": {
"date-parts": [
[
2020,
7,
31
]
]
},
"published-print": {
"date-parts": [
[
2020,
7,
31
]
]
},
"publisher": "American Association for the Advancement of Science (AAAS)",
"reference": [
{
"DOI": "10.1038/s41591-020-0820-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_2_2"
},
{
"DOI": "10.1128/JVI.02615-14",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_3_2"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_4_2"
},
{
"key": "e_1_3_2_5_2",
"unstructured": "L. Cantuti-Castelvetri . bioRxiv 2020.06.07.137802 (2020)."
},
{
"DOI": "10.1007/s00109-006-0094-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_6_2"
},
{
"key": "e_1_3_2_7_2",
"unstructured": "Y. J. Hou . Cell 10.1016/j.cell.2020.05.042 (2020)."
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_8_2"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_9_2"
},
{
"DOI": "10.1126/science.abb2507",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_10_2"
},
{
"DOI": "10.1148/radiol.2020200463",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_11_2"
},
{
"DOI": "10.1126/science.abb7314",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_12_2"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_13_2"
},
{
"DOI": "10.1016/j.trsl.2020.04.007",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_14_2"
},
{
"DOI": "10.1038/s41577-020-0343-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_2_15_2"
},
{
"key": "e_1_3_2_16_2",
"unstructured": "J. Chen . J. Infect. 10.1016/j.jinf.2020.03.004 (2020)."
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.science.org/doi/10.1126/science.abc6156"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "How does SARS-CoV-2 cause COVID-19?",
"type": "journal-article",
"volume": "369"
}