Alteration in liver function tests among patients hospitalized for COVID-19: a multicentric study in Peru
et al., Annals of Hepatology, doi:10.1016/j.aohep.2021.100391, Sep 2021
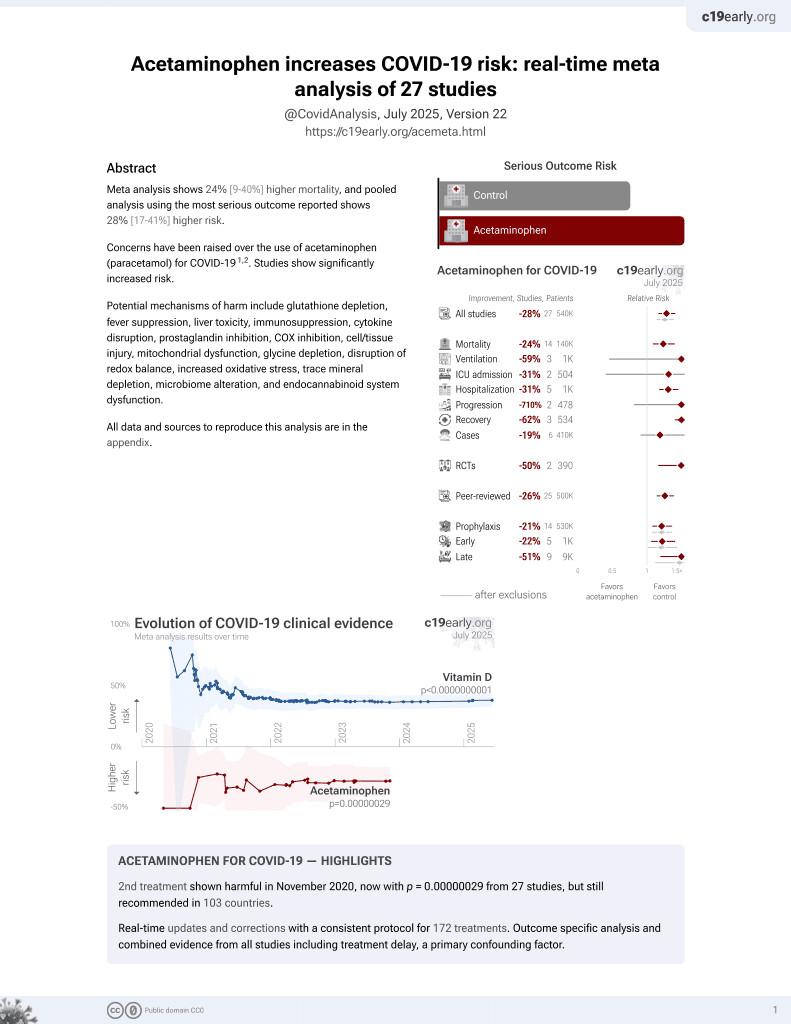
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
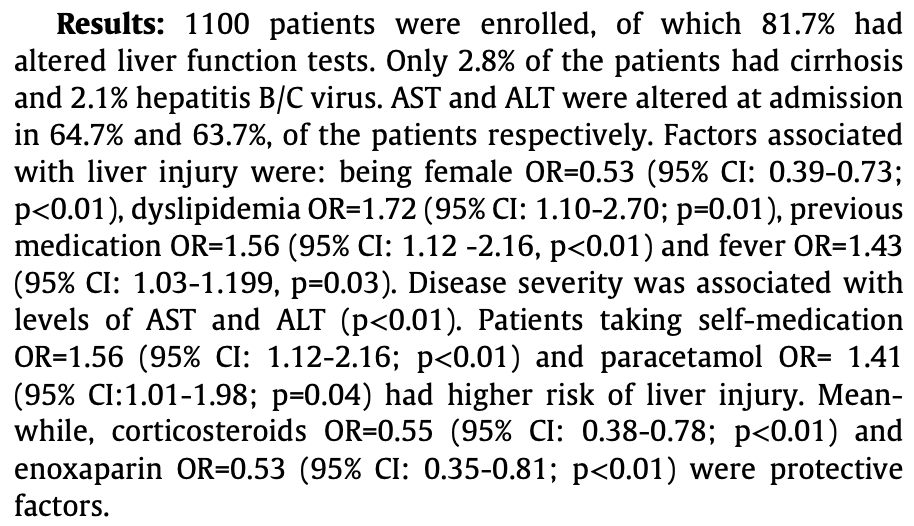
Retrospective 1,100 COVID-19 patients in Peru, showing higher risk of liver injury with paracetamol use.
Paracetamol is also known as acetaminophen, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of liver injury, 41.0% higher, OR 1.41, p = 0.045, liver injury, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Marín Dueñas et al., 30 Sep 2021, retrospective, Peru, peer-reviewed, median age 31.5, 10 authors.
Abstract: Abstracts
P-25 SLOWER FIBROSIS PROGRESSION IN
HEPATITIS C HEMOPHILIAC PATIENTS?
Dauana O. Bastos, Christini Emori, Sandra V. Antunes,
Flavia Appel, Maria Lucia Ferraz
~o Paulo, Sa
~o Paulo, Brazil
Universidade Federal de Sa
Introduction: The improvement in the treatment of hemophilia
from the 900 s, as well as the advent of interferon-free therapy against
HCV enabled a better evolution of these special group of patients.
However, the impact of hemophilia on the progression of liver fibrosis is still not completely understood.
Objectives: To evaluate the progression of liver fibrosis in hemophiliac patients with HCV using non-invasive methods after ten years
of follow-up.
Casuistic and Method: Retrospective cohort study of hemophiliac
patients with HCV evaluated in 2007 and reassessed 10 years later
(2017/2018). Hepatic fibrosis was indirectly evaluated by APRI and
transient hepatic elastography by Fibroscan Ò - EHT).
Results: Sixty-six hemophiliac patients were evaluated in 2007
(all men, median age 31.5 years, 87.9% hemophilia A). Forty-two
patients could be reevaluated in 2017/2018. Thirty-three patients
were treated with 90.9% SVR; thus, after 10 years, 30 patients were
non-viremic and 12 were viremic (3 without SVR and 9 untreated).
APRI values were low in both periods but showed a significant reduction in treated patients (0.36 vs 0.20, p <0.001), remaining stable in
non-treated (0,61 vs 0,51, p=NS). Fibrosis by EHT was assessed only
in 2017/2018 and also showed results compatible with low stages of
fibrosis in treated and even in non-treated patients (4.75 and 5.25
kPa, respectively).
Conclusions: After ten years of follow-up the results suggest a
slower progression and a more benign evolution of hepatic fibrosis
among hemophiliacs. Antiviral therapy against HCV showed an elevated response rate, similar to the general population.
https://doi.org/10.1016/j.aohep.2021.100389
P-26 PORTAL VENOUS THROMBOSIS IN
TRANSPLANTED CIRRHOTIC PATIENTS AT THE
“HOSPITAL CLINICO UNIVERSIDAD DE CHILE”
V.N. Henríquez Auba1, F. Ramirez1, M. Fabres2,
P. Abarca2, B. Astrosa2, D. Vera1, J.C. Diaz3, J. Castillo3,
3, M. Cattaneo1, J. Poniachik1,3,
H. Lembach3, A. Saure
A. Urzua1,3
n de Gastroenterología,
Universidad de Chile, Seccio
Santiago, Chile
2
Universidad de Chile, Santiago, Chile
3
Universidad de Chile, Unidad de Trasplantes,
Santiago, Chile
1
Background: Portal vein thrombosis (PVT) is a frequent complication in cirrhotic patients on the waiting list for liver transplantation
(LT); this is associated with increased post-LT mortality.
Objective: Characterize the presence of PVT in patients with LT.
Methods: Retrospective observational study between January 1,
2014 and February 28, 2018. Clinical records, laboratory and images
were reviewed.
Results: 82 patients were included; Age 58 (21-71) years; Etiology: non-alcoholic fatty liver 40.2%, alcoholic liver disease 20.7%,
autoimmunity 13.4%, and hepatitis C 8.5%; Child-Pugh: 7.3% A, 30.4%
B and 62.2% C; MELD-Na 22 (8-40). PVT was diagnosed before or during LT in 26.8%: Child A 16.6%, B 16.0%, and C 33.3%; MELD-Na 25 (1240) in those with PVT vs 21 (8-40) in those without PVT (non significant, NS); 34% had hepatocarcinoma (32.1% with PVT vs. 24.4%
Annals of Hepatology 24 (2021) 100366
without PVT; NS). Diagnosis of PVT was 77.2% pre LT and almost 1/4
during transplant surgery. The extension of the PVT was complete
occlusion in 11.7%, partial in 70.5%; 11.7% had only intrahepatic
branches compromised..
DOI record:
{
"DOI": "10.1016/j.aohep.2021.100391",
"ISSN": [
"1665-2681"
],
"URL": "http://dx.doi.org/10.1016/j.aohep.2021.100391",
"alternative-id": [
"S1665268121000909"
],
"article-number": "100391",
"author": [
{
"affiliation": [],
"family": "Marín Dueñas",
"given": "Ivania",
"sequence": "first"
},
{
"affiliation": [],
"family": "Vega",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrillo-Ng",
"given": "Hugo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veramendi-Schult",
"given": "Isabel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zavaleta Alva",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vásquez",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gonzales-Soler",
"given": "Zeneida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agurto-Lescano",
"given": "Hellen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garavito Rentería",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lozano",
"given": "Adelina",
"sequence": "additional"
}
],
"container-title": "Annals of Hepatology",
"container-title-short": "Annals of Hepatology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
27
]
],
"date-time": "2021-09-27T13:44:49Z",
"timestamp": 1632750289000
},
"deposited": {
"date-parts": [
[
2021,
11,
5
]
],
"date-time": "2021-11-05T00:15:24Z",
"timestamp": 1636071324000
},
"indexed": {
"date-parts": [
[
2022,
3,
30
]
],
"date-time": "2022-03-30T13:32:23Z",
"timestamp": 1648647143392
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
22
]
],
"date-time": "2021-07-22T00:00:00Z",
"timestamp": 1626912000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1665268121000909?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1665268121000909?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100391",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1665268121000909"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Hepatology",
"General Medicine"
],
"subtitle": [],
"title": "P-27 ALTERATION IN LIVER FUNCTION TESTS AMONG PATIENTS HOSPITALIZED FOR COVID-19: A MULTICENTRIC STUDY IN PERU",
"type": "journal-article",
"volume": "24"
}