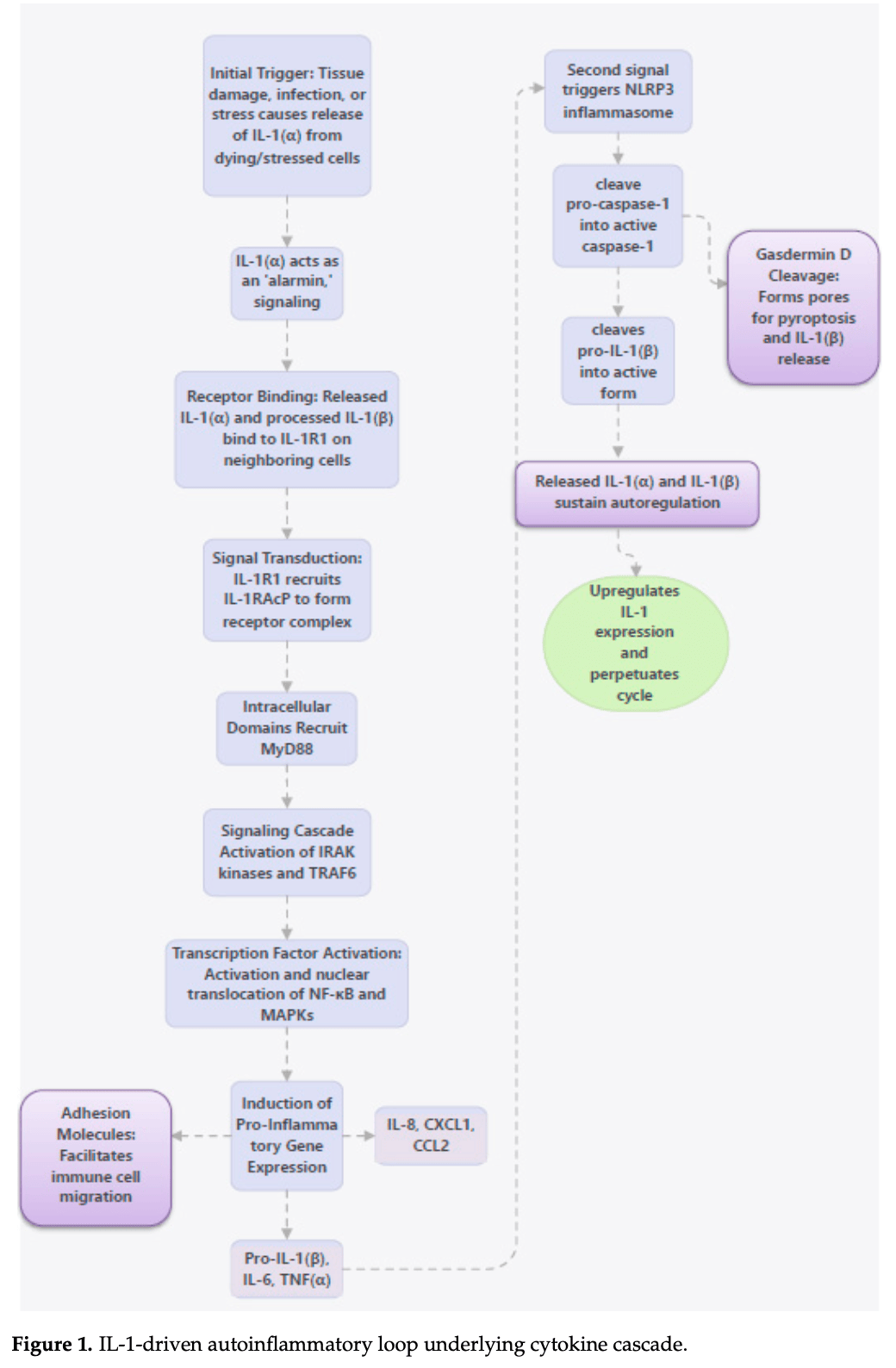
Interleukins in COVID-19 and SARS-CoV-2 Variants: Immunopathogenesis, Therapeutic Perspectives and Vaccine-Induced Immune Responses
et al., International Journal of Molecular Sciences, doi:10.3390/ijms27031391, Jan 2026
Review of the role of interleukins in COVID-19 immunopathogenesis and therapeutic approaches.
See Xie et al. for another review covering tocilizumab for COVID-19.
Mahajan et al., 30 Jan 2026, peer-reviewed, 3 authors.
Contact: drsupriyamahajan@gmail.com (corresponding author).
Interleukins in COVID-19 and SARS-CoV-2 Variants: Immunopathogenesis, Therapeutic Perspectives and Vaccine-Induced Immune Responses
International Journal of Molecular Sciences, doi:10.3390/ijms27031391
The Coronavirus disease 2019 , caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by profound immune dysregulation where interleukins play a central role in determining disease severity and response to interventions. This review summarizes the role of interleukins in the immunopathogenesis of COVID-19, with particular emphasis on differences observed across major SARS-CoV-2 variants. Pro-inflammatory interleukins like IL-1β, IL-6, IL-2, IL-17 and IL-18 are critically involved in cytokine storm, hyperinflammation, and acute respiratory distress syndrome, whereas anti-inflammatory cytokines like IL-10 contribute to immune regulation and resolution of inflammation. Elevated levels of IL-1α, IL-1β, IL-4, IL-8, IL-9, IL-16, IL-18 have been documented in the Delta variant as compared with the Omicron variant, with IL-6 being the most frequent interleukin reported to be increased across all SARS-CoV-2 variants relative to the ancestral Wuhan strain. Elevated IL-2, IL-4, IL-6, and IL-10 levels have been associated with Omicron sub-variants. The review encompasses interleukin-based therapeutic strategies, where several IL-1 and IL-6 inhibitors were studied across clinical trials, but only tocilizumab has shown some promise against severe COVID-19. IL-2, IL-6, IL-15 and IL-21 levels were positively correlated with IgG and neutralizing antibody activity after vaccination with longevity of post-vaccination immunity being determined by IL-2 and IL-7.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Abbas, Lichtman, Pillai, Cytokines, Cellular and Molecular Immunology
Abbood, Anvari, Fateh, Association between interleukin-10 gene polymorphisms (rs1800871, rs1800872, and rs1800896) and severity of infection in different SARS-CoV-2 variants, Hum. Genom, doi:10.1186/s40246-023-00468-6
Al-Qahtani, Alhamlan, Al-Qahtani, Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review, Trop. Med. Infect. Dis, doi:10.3390/tropicalmed9010013
Albayrak, Orte Cano, Karimi, Dogahe, Van Praet et al., Distinct Expression Patterns of Interleukin-22 Receptor 1 on Blood Hematopoietic Cells in SARS-CoV-2 Infection, Front. Immunol, doi:10.3389/fimmu.2022.769839
Alcorn, IL-22 Plays a Critical Role in Maintaining Epithelial Integrity During Pulmonary Infection, Front. Immunol, doi:10.3389/fimmu.2020.01160
Antoniv, Ivashkiv, Dysregulation of interleukin-10-dependent gene expression in rheumatoid arthritis synovial macrophages, Arthritis Rheum, doi:10.1002/art.22055
Avdeev, Trushenko, Tsareva, Yaroshetskiy, Merzhoeva et al., Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study, Cytokine, doi:10.1016/j.cyto.2021.155627
Azaiz, Jemaa, Sellami, Romdhani, Ouslati et al., Deciphering the balance of IL-6/IL-10 cytokines in severe to critical COVID-19 patients, Immunobiology, doi:10.1016/j.imbio.2022.152236
Barata, Durum, Seddon, Flip the coin: IL-7 and IL-7R in health and disease, Nat. Immunol, doi:10.1038/s41590-019-0479-x
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps, J. Exp. Med, doi:10.1084/jem.20200652
Barreto, Cruz, Volle, Júnior, Costa et al., Clinical Manifestations and Cytokine Profiles of the Th1, Th2, and Th17 Response Associated with SARS-CoV-2 Omicron Subvariants, Biomedicines, doi:10.3390/biomedicines13092128
Bekele, Sui, Berzofsky, IL-7 in SARS-CoV-2 Infection and as a Potential Vaccine Adjuvant, Front. Immunol, doi:10.3389/fimmu.2021.737406
Bergamaschi, Terpos, Rosati, Angel, Bear et al., Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients, Cell Rep, doi:10.1016/j.celrep.2021.109504
Bhaumik, Basu, Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response, Front. Immunol, doi:10.3389/fimmu.2017.00254
Blom, Poulsen, IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures, J. Immunol, doi:10.4049/jimmunol.1103685
Bostanghadiri, Ziaeefar, Mofrad, Yousefzadeh, Hashemi et al., COVID-19: An Overview of SARS-CoV-2 Variants-The Current Vaccines and Drug Development, Biomed Res. Int, doi:10.1155/2023/1879554
Burke, Freeman, Cellura, Stuart, Brendish et al., Inflammatory phenotyping predicts clinical outcome in COVID-19, Respir. Res, doi:10.1186/s12931-020-01511-z
Calvo-Alvarez, D'alessandro, Zanotta, Basilico, Parapini et al., Multiplex array analysis of circulating cytokines and chemokines in COVID-19 patients during the first wave of the SARS-CoV-2 pandemic in Milan, Italy, BMC Immunol, doi:10.1186/s12865-024-00641-z
Cao, Wang, Liu, Liu, Li et al., Helminth alleviates COVID-19-related cytokine storm in an IL-9-dependent way, mBio, doi:10.1128/mbio.00905-24
Cavalli, De Luca, Campochiaro, Della-Torre, Ripa et al., Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study, Lancet Rheumatol, doi:10.1016/S2665-9913(20)30127-2
Chang, Bai, You, Associations between Serum Interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis, Biomed Res. Int, doi:10.1155/2022/2755246
Conti, Ronconi, Caraffa, Gallenga, Ross et al., Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies, J. Biol. Regul. Homeost. Agents
Coomes, Haghbayan, Interleukin-6 in Covid-19: A systematic review and meta-analysis, Rev. Med. Virol, doi:10.1002/rmv.2141
Davidson, Menon, Chaimani, Evrenoglou, Ghosn et al., Interleukin-1 blocking agents for treating COVID-19, Cochrane Database Syst. Rev
Dhar, Vishnupriyan, Damodar, Gujar, Das, IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression, Heliyon, doi:10.1016/j.heliyon.2021.e06155
Di Spigna, Covelli, Vargas, Di Caprio, Rubino et al., The Behaviour of IL-6 and Its Soluble Receptor Complex during Different Waves of the COVID-19 Pandemic, Life, doi:10.3390/life14070814
Dinarello, The IL-1 family and inflammatory diseases, Clin. Exp. Rheumatol
Elkassar, Gress, An overview of IL-7 biology and its use in immunotherapy, J. Immunotoxicol, doi:10.3109/15476910903453296
Fang, Ju, Lin, Chen, The role of interleukin-22 in lung health and its therapeutic potential for COVID-19, Front. Immunol, doi:10.3389/fimmu.2022.951107
Fonseca, Asai, Yagi, Malinczak, Savickas et al., COVID-19 Modulates Inflammatory and Renal Markers That May Predict Hospital Outcomes among African American Males, Viruses, doi:10.3390/v13122415
Gao, Cai, Li, Zhang, Li et al., Emerging Effects of IL-33 on COVID-19, Int. J. Mol. Sci, doi:10.3390/ijms232113656
García, Kokkinou, Carrasco García, Parrot, Palma Medina et al., Innate lymphoid cell composition associates with COVID-19 disease severity, Clin. Transl. Immunol, doi:10.1002/cti2.1224
Ghanbari Naeini, Abbasi, Karimi, Kokabian, Abdi Abyaneh et al., The Important Role of Interleukin-2 in COVID-19, J. Immunol. Res, doi:10.1155/2023/7097329
Ghazavi, Ganji, Keshavarzian, Rabiemajd, Mosayebi, Cytokine profile and disease severity in patients with COVID-19, Cytokine, doi:10.1016/j.cyto.2020.155323
Ghofrani Nezhad, Jami, Kooshkaki, Chamani, Naghizadeh, The Role of Inflammatory Cytokines (Interleukin-1 and Interleukin-6) as a Potential Biomarker in the Different Stages of COVID-19 (Mild, Severe, and Critical), J. Interferon Cytokine Res, doi:10.1089/jir.2022.0185
Ghosn, Assi, Evrenoglou, Buckley, Henschke et al., Current Evidence of Interleukin-6 Signaling Inhibitors in Patients with COVID-19: A Systematic Review and Meta-Analysis, Cochrane Database Syst. Rev, doi:10.3389/fphar.2020.615972
Gubernatorova, Gorshkova, Polinova, Drutskaya, IL-6: Relevance for immunopathology of SARS-CoV-2, Cytokine Growth Factor Rev, doi:10.1016/j.cytogfr.2020.05.009
Hamdy, Elhamammy, Abdelmageed, Wahid, Impact of single nucleotide polymorphism of IL-27P28 rs153109 and IFITM3 rs12252 on susceptibility and severity of COVID-19 in Egyptian patients: A case control study, Virol. J, doi:10.1186/s12985-025-02668-z
Han, Ma, Li, Liu, Zhao et al., Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1770129
Hasanvand, COVID-19 and the role of cytokines in this disease, Inflammopharmacology, doi:10.1007/s10787-022-00992-2
Hurme, Jalkanen, Heroum, Liedes, Vara et al., Long-Lasting T Cell Responses in BNT162b2 COVID-19 mRNA Vaccinees and COVID-19 Convalescent Patients, Front. Immunol
Irvem, Erdogan Cakir, Aydın, Mart Komurcu, Celik et al., Effect of SARS-CoV-2 variants (Alpha, Beta, Delta, Omicron) on inflammatory parameters, Jundishapur J. Microbiol, doi:10.5812/jjm-159675
Islam, Chamberlain, Mui, Little, Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front, Immunol, doi:10.3389/fimmu.2021.677008
Jamilloux, Henry, Belot, Viel, Fauter et al., Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102567
Ji, Tassiulas, Park-Min, Aydin, Mecklenbrauker et al., Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis, J. Exp. Med, doi:10.1084/jem.20021820
Jiang, Wang, Yao, Lai, Zhang, What do we know about IL-6 in COVID-19 so far?, Biophys. Rep, doi:10.52601/bpr.2021.200024
Joo, Kim, Hak Choi, Han, Lee et al., Increased expression of interleukin 36 in chronic rhinosinusitis and its contribution to chemokine secretion and increased epithelial permeability, Cytokine
Junqueira, Crespo, Ranjbar, Ingber, Parry et al., SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release, medRxiv, doi:10.1101/2021.03.06.21252796
Kakugawa, Mimura, Mimura-Kimura, Doi, Ohteru et al., Kinetics of pro-and anti-inflammatory spike-specific cellular immune responses in long-term care facility residents after COVID-19 mRNA primary and booster vaccination: A prospective longitudinal study in Japan, Immun. Ageing, doi:10.1186/s12979-024-00444-1
Kawasuji, Morinaga, Nagaoka, Tani, Yoshida et al., High interleukin-6 levels induced by COVID-19 pneumonia correlate with increased circulating follicular helper T cell frequency and strong neutralization antibody response in the acute phase of Omicron breakthrough infection, Front. Immunol, doi:10.3389/fimmu.2024.1377014
Kindler, Thiel, To sense or not to sense viral RNA-Essentials of coronavirus innate immune evasion, Curr. Opin. Microbiol, doi:10.1016/j.mib.2014.05.005
Klooster, Bol-Schoenmakers, Van Summeren, Van Vliet, De Haan et al., fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine, Commun. Biol, doi:10.1038/s42003-021-02176-0
Korobova, Arsentieva, Liubimova, Batsunov, Dedkov et al., Cytokine Profiling in Different SARS-CoV-2 Genetic Variants, Int. J. Mol. Sci, doi:10.3390/ijms232214146
Korobova, Arsentieva, Santoni, Totolian, Role of IL-27 in COVID-19: A Thin Line between Protection and Disease Promotion, Int. J. Mol. Sci, doi:10.3390/ijms25147953
Koutsakos, Lee, Wheatley, Kent, Juno, T follicular helper cells in the humoral immune response to SARS-CoV-2 infection and vaccination, J. Leukoc. Biol, doi:10.1002/JLB.5MR0821-464R
Krivosova, Hanusrichterova, Lucansky, Samec, Bobcakova et al., Comparative study of cytokine profiles in SARS-CoV-2 Delta and Omicron variants, Bratisl. Med. J, doi:10.1007/s44411-024-00010-7
Landi, Ravaglia, Russo, Cataleta, Fusari et al., Blockage of interleukin-1β with canakinumab in patients with Covid-19, Sci. Rep, doi:10.1038/s41598-020-78492-y
Lariccia, Magi, Serfilippi, Toujani, Gratteri et al., Challenges and Opportunities from Targeting Inflammatory Responses to SARS-CoV-2 Infection: A Narrative Review, J. Clin. Med, doi:10.3390/jcm9124021
Laterre, François, Collienne, Hantson, Jeannet et al., Association of Interleukin 7 Immunotherapy with Lymphocyte Counts Among Patients with Severe Coronavirus Disease 2019 (COVID-19), JAMA Netw. Open, doi:10.1001/jamanetworkopen.2020.16485
Li, Li, Liu, Kang, Zhang et al., A comparative study of spike protein of SARS-CoV-2 and its variant Omicron (B.1.1.529) on some immune characteristics, Sci. Rep, doi:10.1038/s41598-022-21690-7
Li, Ren, Cao, Roles of Interleukin-6-mediated immunometabolic reprogramming in COVID-19 and other viral infection-associated diseases, Int. Immunopharmacol, doi:10.1016/j.intimp.2022.109005
Li, Wang, Jin, Li, Yan et al., A comprehensive analysis of immune characteristics and clinical prognosis in Asian COVID-19 patients infected with SARS-CoV-2 Omicron strain XBB sub-variants: A retrospective study of 450 cases, Arch. Med. Sci, doi:10.5114/aoms/178422
Liang, Bao, Yang, Liu, Sun et al., SARS-CoV-2 spike protein induces IL-18-mediated cardiopulmonary inflammation via reduced mitophagy, Signal Transduct. Target. Ther
Liang, Li, Meng, Li, Mai et al., Prognostic significance of serum interleukin-6 in severe/critical COVID-19 patients treated with tocilizumab: A detailed observational study analysis
Liu, Dwyer, Zhao, Li, Mathews et al., IL-33-mediated IL-13 secretion by ST2+ Tregs controls inflammation after lung injury, JCI Insight, doi:10.1172/jci.insight.123919
Liu, Guo, Zhan, Liu, Li et al., Immune and inflammation features of severe and critical Omicron infected patients during Omicron wave in China, BMC Infect. Dis, doi:10.1186/s12879-024-09652-y
Makaremi, Asgarzadeh, Kianfar, Mohammadnia, Asghariazar et al., The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19, Inflamm. Res, doi:10.1007/s00011-022-01596-w
Malahe, Hartog, Rietdijk, Van Baarle, De Kuiper et al., The role of interleukin-21 in COVID-19 vaccine-induced B cell-mediated immune responses in patients with kidney disease and kidney transplant recipients, Am. J. Transplant, doi:10.1016/j.ajt.2023.05.025
Marino, Criniti, Guida, Bucci, Ballesio et al., Interleukin 18 and IL-18 BP response to Sars-CoV-2 virus infection, Clin. Exp. Med, doi:10.1007/s10238-022-00943-9
Martinon, Burns, Tschopp, The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta, Mol. Cell, doi:10.1016/S1097-2765(02)00599-3
Martonik, Parfieniuk-Kowerda, Rogalska, Flisiak, The Role of Th17 Response in COVID-19, Cells, doi:10.3390/cells10061550
Mazer, Turnbull, Miles, Blood, Sadler et al., Interleukin-7 Reverses Lymphopenia and Improves T-Cell Function in Coronavirus Disease 2019 Patient with Inborn Error of Toll-Like Receptor 3: A Case Report, Crit. Care Explor, doi:10.1097/CCE.0000000000000500
Mcgeachy, Cua, Gaffen, The IL-17 Family of Cytokines in Health and Disease, Immunity, doi:10.1016/j.immuni.2019.03.021
Mcgonagle, Sharif, O'regan, Bridgewood, The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease, Autoimmun. Rev, doi:10.1016/j.autrev.2020.102537
Meka, Venkatesha, Dudics, Acharya, Moudgil, IL-27-induced modulation of autoimmunity and its therapeutic potential, Autoimmun. Rev, doi:10.1016/j.autrev.2015.08.001
Mendoza, Interleukin-17: A potential therapeutic target in COVID-19, J. Infect, doi:10.1016/j.jinf.2020.05.072
Merchant, Ashraf, Masood, Yameen, Hussain et al., SARS-CoV-2 variants induce increased inflammatory gene expression but reduced interferon responses and heme synthesis as compared with wild type strains, Sci. Rep, doi:10.1038/s41598-024-76401-1
Mercurio, Failla, Capriotti, Scarponi, Facchiano et al., Interleukin (IL)-17/IL-36 axis participates to the crosstalk between endothelial cells and keratinocytes during inflammatory skin responses, PLoS ONE, doi:10.1371/journal.pone.0222969
Monteil, Kwon, Prado, Hagelkrüys, Wimmer et al., Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Najafi-Fard, Petruccioli, Farroni, Petrone, Vanini et al., Evaluation of the immunomodulatory effects of interleukin-10 on peripheral blood immune cells of COVID-19 patients: Implication for COVID-19 therapy, Front. Immunol, doi:10.3389/fimmu.2022.984098
Nakanishi, Unique Action of Interleukin-18 on T Cells and Other Immune Cells, Front. Immunol, doi:10.3389/fimmu.2018.00763
Niu, Liang, Chen, Zhu, Zhou et al., SARS-CoV-2 spike protein induces the cytokine release syndrome by stimulating T cells to produce more IL-2, Front. Immunol, doi:10.3389/fimmu.2024.1444643
Orlov, Wander, Morrell, Mikacenic, Wurfel, A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections, J. Immunol, doi:10.4049/jimmunol.2000554
Paranga, Mitu, Pavel-Tanasa, Rosu, Miftode et al., Cytokine Storm in COVID-19: Exploring IL-6 Signaling and Cytokine-Microbiome Interactions as Emerging Therapeutic Approaches, Int. J. Mol. Sci, doi:10.3390/ijms252111411
Pierce, Sy, Galen, Goldstein, Orner et al., Natural mucosal barriers and COVID-19 in children, JCI Insight, doi:10.1172/jci.insight.148694
Plassmeyer, Alpan, Corley, Premeaux, Lillard et al., Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS-CoV-2 infection and long COVID, Allergy, doi:10.1111/all.14907
Pociask, Scheller, Mandalapu, Mchugh, Enelow et al., IL-22 is essential for lung epithelial repair following influenza infection, Am. J. Pathol, doi:10.1016/j.ajpath.2012.12.007
Potere, Batticciotto, Vecchié, Porreca, Cappelli et al., The role of IL-6 and IL-6 blockade in COVID-19, Expert. Rev. Clin. Immunol, doi:10.1080/1744666X.2021.1919086
Rahimi, Bezmin Abadi, WHO prequalified tocilizumab and vaccine boosters against COVID-19, Int. J. Surg, doi:10.1016/j.ijsu.2022.106593
Rajamanickam, Nathella, Selvaraj, Manoj, Thangaraj et al., Characterization of IL-10 Family of Cytokines in Acute and Convalescent COVID-19 Individuals, J. Interferon Cytokine Res, doi:10.1089/jir.2023.0075
Raman, Patel, Ranjan, COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies, Biomolecules, doi:10.3390/biom11070993
Resende, Da Cruz Lage, Lobê, Medeiros, Costa Esilva et al., Blockade of interleukin seventeen (IL-17A) with secukinumab in hospitalized COVID-19 patients-The BISHOP study, Infect. Dis, doi:10.1080/23744235.2022.2066171
Retnakumar, Singh, Chauvin, Bayry, IL-33 and IL-3 synergistically induce CD25 expression on human basophils without functional IL-2 signaling: A potential marker of severe COVID-19, Front. Immunol, doi:10.3389/fimmu.2025.1718240
Reuschl, Thorne, Whelan, Ragazzini, Furnon et al., Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants, Nat. Microbiol, doi:10.1038/s41564-023-01588-4
Rodrigues, De Sá, Ishimoto, Becerra, Oliveira et al., Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients, J. Exp. Med, doi:10.1084/jem.20201707
Rodríguez-Morales, Guartazaca-Guerrero, Rizo-Téllez, Viurcos-Sanabria, Barrón et al., Blood-brain Barrier Damage is Pivotal for SARS-CoV-2 Infection to the Central Nervous System, Exp. Neurobiol, doi:10.5607/en21049
Sadhu, Dalal, Dandotiya, Binayke, Singh et al., IL-9 aggravates SARS-CoV-2 infection and exacerbates associated airway inflammation, Nat. Commun, doi:10.1038/s41467-023-39815-5
Santos, Almeida, Coelho, Freire, Silva et al., Anti-inflammatory interleukins (IL-4 and IL-10) and their relationship with the severity of COVID-19: A systematic review, Res. Soc. Dev, doi:10.33448/rsd-v14i3.48459
Saraiva, Vieira, O'garra, Biology and therapeutic potential of interleukin-10, J. Exp. Med, doi:10.1084/jem.20190418
Satış, Özger, Aysert Yıldız, Hızel, Gulbahar et al., Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19, Cytokine, doi:10.1016/j.cyto.2020.155302
Schooling, Li, Au Yeung, Interleukin-18 and COVID-19, Epidemiol. Infect, doi:10.1017/S0950268821002636
Shahbaz, Bozorgmehr, Lu, Osman, Sligl et al., Analysis of SARS-CoV-2 isolates, namely the Wuhan strain, Delta variant, and Omicron variant, identifies differential immune profiles, Microbiol. Spectr, doi:10.1128/spectrum.01256-23
Shankar-Hari, Francois, Remy, Gutierrez, Pastores et al., A randomized, double-blind, placebo-controlled trial of IL-7 in critically ill patients with COVID-19, JCI Insight, doi:10.1172/jci.insight.189150
Sharif-Askari, Sharif-Askari, Hafezi, Mdkhana, Alsayed et al., Interleukin-17, a salivary biomarker for COVID-19 severity, PLoS ONE, doi:10.1371/journal.pone.0274841
Shi, Gao, Shao, Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death, Trends Biochem. Sci, doi:10.1016/j.tibs.2016.10.004
Shi, Wang, Yin, Ouyang, Pang et al., The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia, Cell Death Dis, doi:10.1038/s41419-020-2636-4
Shih, Yang, Liao, Lu, Hu et al., An important call: Suggestion of using IL-10 as therapeutic agent for COVID-19 with ARDS and other complications, Virulence, doi:10.1080/21505594.2023.2190650
Siedlecka, Bielawska, Ludziejewska, Baszczuk, Wysocka, IL-2 and IL-7 Contribution to Immune Response: Effects of Vaccination Against COVID-19 in Adults, Viruses, doi:10.3390/v17111416
Singh, Hemati, Bajpai, Yadav, Maheshwari et al., Sustained expression of inflammatory monocytes and activated T cells in COVID-19 patients and recovered convalescent plasma donors, Immun. Inflamm. Dis, doi:10.1002/iid3.476
Slaats, Ten Oever, Van De Veerdonk, Netea, IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections, PLoS Pathog, doi:10.1371/journal.ppat.1005973
Spracklen, Mendelsohn, Butters, Facey-Thomas, Stander et al., IL27 gene expression distinguishes multisystem inflammatory syndrome in children from febrile illness in a South African cohort, Front. Immunol, doi:10.3389/fimmu.2022.992022
Stanczak, Sanin, Apostolova, Nerz, Lampaki et al., IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals, Nat. Commun, doi:10.1038/s41467-021-22449-w
Stebbing, Krishnan, De Bono, Ottaviani, Casalini et al., Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients, EMBO Mol. Med, doi:10.15252/emmm.202012697
Tao, Zhang, Wang, Guo, Zeng et al., Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18, Med. Microecol, doi:10.1016/j.medmic.2020.100023
Theobald, Simonis, Georgomanolis, Kreer, Zehner et al., Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19, EMBO Mol. Med, doi:10.15252/emmm.202114150
Thys, Loza, Lynn, Callewaert, Varma et al., Pharmacodynamic, prognostic, and predictive biomarkers in severe and critical COVID-19 patients treated with sirukumab, Sci. Rep, doi:10.1038/s41598-024-74196-9
Tjan, Furukawa, Nagano, Kiriu, Nishimura et al., Early Differences in Cytokine Production by Severity of Coronavirus Disease, J. Infect. Dis, doi:10.1093/infdis/jiab005
Udomsinprasert, Jittikoon, Sangroongruangsri, Chaikledkaew, Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis, J. Clin. Immunol, doi:10.1007/s10875-020-00899-z
Valdés-López, Urcuqui-Inchima, Antiviral response and immunopathogenesis of interleukin 27 in COVID-19, Arch. Virol, doi:10.1007/s00705-023-05792-9
Van De Veerdonk, Netea, Blocking IL-1 to prevent respiratory failure in COVID-19, Crit. Care, doi:10.1186/s13054-020-03166-0
Wang, Tang, Liu, Zhang, Chen et al., The role of IL-6 in coronavirus, especially in COVID-19, Front. Pharmacol, doi:10.3389/fphar.2022.1033674
Wang, Wang, Zheng, Zhang, Wang et al., Identification of an IL-1 receptor mutation driving autoinflammation directs IL-1-targeted drug design, Immunity, doi:10.1016/j.immuni.2023.05.014
Wang, Yi, Liang, The Role of IL-36 in Infectious Diseases: Potential Target for COVID-19?, Front. Immunol, doi:10.3389/fimmu.2021.662266
Waseem, Shariff, Lim, Nunez, Narayanan et al., Multisystem Inflammatory Syndrome in Children, West. J. Emerg. Med, doi:10.5811/westjem.2022.3.55325
Williams, O'callaghan, Corr, IL-33 and IL-18 in Inflammatory Bowel Disease Etiology and Microbial Interactions, Front. Immunol, doi:10.3389/fimmu.2019.01091
Wilson, Madala, Ramalingam, Gochuico, Rosas et al., Bleomycin and IL-1betamediated pulmonary fibrosis is IL-17A dependent, J. Exp. Med, doi:10.1084/jem.20092121
Wu, Yang, TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib, J. Microbiol. Immunol. Infect, doi:10.1016/j.jmii.2020.03.005
Xiang, Zhong, Wang, IL-9 plays a critical role in helminth-induced protection against COVID-19-related cytokine storms, mBio, doi:10.1128/mbio.01229-24
Xu, Guardado, Hoffman, Xu, Namas et al., IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model, PLoS Med, doi:10.1371/journal.pmed.1002365
Zhang, Hao, Ou, Ming, Liang et al., Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study, J. Transl. Med, doi:10.1186/s12967-020-02571-x
Zhu, Pang, Ji, Zhong, Li et al., Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis, J. Med. Virol, doi:10.1002/jmv.26085
Zizzo, Tamburello, Castelnovo, Laria, Mumoli et al., Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling, Front. Immunol, doi:10.3389/fimmu.2022.795315
Zulli, Burrell, Buxton, Hare, ACE2 and AT4R are present in diseased human blood vessels, Eur. J. Histochem, doi:10.4081/1184
DOI record:
{
"DOI": "10.3390/ijms27031391",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms27031391",
"abstract": "<jats:p>The Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by profound immune dysregulation where interleukins play a central role in determining disease severity and response to interventions. This review summarizes the role of interleukins in the immunopathogenesis of COVID-19, with particular emphasis on differences observed across major SARS-CoV-2 variants. Pro-inflammatory interleukins like IL-1β, IL-6, IL-2, IL-17 and IL-18 are critically involved in cytokine storm, hyperinflammation, and acute respiratory distress syndrome, whereas anti-inflammatory cytokines like IL-10 contribute to immune regulation and resolution of inflammation. Elevated levels of IL-1α, IL-1β, IL-4, IL-8, IL-9, IL-16, IL-18 have been documented in the Delta variant as compared with the Omicron variant, with IL-6 being the most frequent interleukin reported to be increased across all SARS-CoV-2 variants relative to the ancestral Wuhan strain. Elevated IL-2, IL-4, IL-6, and IL-10 levels have been associated with Omicron sub-variants. The review encompasses interleukin-based therapeutic strategies, where several IL-1 and IL-6 inhibitors were studied across clinical trials, but only tocilizumab has shown some promise against severe COVID-19. IL-2, IL-6, IL-15 and IL-21 levels were positively correlated with IgG and neutralizing antibody activity after vaccination with longevity of post-vaccination immunity being determined by IL-2 and IL-7.</jats:p>",
"alternative-id": [
"ijms27031391"
],
"author": [
{
"affiliation": [
{
"name": "Department of Microbiology, School of Medical Sciences and Research, Sharda University, Greater Noida 201306, UP, India"
}
],
"family": "Mahajan",
"given": "Supriya",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Numed Super Speciality Hospital, Greater Noida 201306, UP, India"
}
],
"family": "Mahajan",
"given": "Saurabh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, School of Medical Sciences and Research, Sharda University, Greater Noida 201306, UP, India"
}
],
"family": "Gusain",
"given": "Akanksha",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T11:09:22Z",
"timestamp": 1769771362000
},
"deposited": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T11:12:09Z",
"timestamp": 1769771529000
},
"indexed": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T21:45:31Z",
"timestamp": 1769809531612,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2026,
1,
30
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2026,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T00:00:00Z",
"timestamp": 1769731200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/27/3/1391/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1391",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2026,
1,
30
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
30
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/j.mib.2014.05.005",
"article-title": "To sense or not to sense viral RNA—Essentials of coronavirus innate immune evasion",
"author": "Kindler",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Curr. Opin. Microbiol.",
"key": "ref_1",
"volume": "20",
"year": "2014"
},
{
"article-title": "The Important Role of Interleukin-2 in COVID-19",
"author": "Abbasi",
"first-page": "7097329",
"journal-title": "J. Immunol. Res.",
"key": "ref_2",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.3390/tropicalmed9010013",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Al-Qahtani, A.A., Alhamlan, F.S., and Al-Qahtani, A.A. (2024). Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis., 9."
},
{
"DOI": "10.1186/s13054-020-03166-0",
"article-title": "Blocking IL-1 to prevent respiratory failure in COVID-19",
"author": "Netea",
"doi-asserted-by": "crossref",
"first-page": "445",
"journal-title": "Crit. Care",
"key": "ref_4",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102567",
"article-title": "Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions",
"author": "Jamilloux",
"doi-asserted-by": "crossref",
"first-page": "102567",
"journal-title": "Autoimmun. Rev.",
"key": "ref_5",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/S1097-2765(02)00599-3",
"article-title": "The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta",
"author": "Martinon",
"doi-asserted-by": "crossref",
"first-page": "417",
"journal-title": "Mol. Cell",
"key": "ref_6",
"volume": "10",
"year": "2002"
},
{
"DOI": "10.1016/j.immuni.2023.05.014",
"article-title": "Identification of an IL-1 receptor mutation driving autoinflammation directs IL-1-targeted drug design",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1485",
"journal-title": "Immunity",
"key": "ref_7",
"volume": "56",
"year": "2023"
},
{
"article-title": "The IL-1 family and inflammatory diseases",
"author": "Dinarello",
"first-page": "S1",
"journal-title": "Clin. Exp. Rheumatol.",
"key": "ref_8",
"volume": "20",
"year": "2002"
},
{
"DOI": "10.1016/j.tibs.2016.10.004",
"article-title": "Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "Trends Biochem. Sci.",
"key": "ref_9",
"volume": "42",
"year": "2017"
},
{
"DOI": "10.1016/S2665-9913(20)30127-2",
"article-title": "Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study",
"author": "Cavalli",
"doi-asserted-by": "crossref",
"first-page": "e325",
"journal-title": "Lancet Rheumatol.",
"key": "ref_10",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.5607/en21049",
"article-title": "Blood-brain Barrier Damage is Pivotal for SARS-CoV-2 Infection to the Central Nervous System",
"author": "Nava",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Exp. Neurobiol.",
"key": "ref_11",
"volume": "31",
"year": "2022"
},
{
"article-title": "Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies",
"author": "Conti",
"first-page": "327",
"journal-title": "J. Biol. Regul. Homeost. Agents",
"key": "ref_12",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiab005",
"article-title": "Early Differences in Cytokine Production by Severity of Coronavirus Disease 2019",
"author": "Tjan",
"doi-asserted-by": "crossref",
"first-page": "1145",
"journal-title": "J. Infect. Dis.",
"key": "ref_13",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1155/2022/2755246",
"article-title": "Associations between Serum Interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis",
"author": "Chang",
"doi-asserted-by": "crossref",
"first-page": "2755246",
"journal-title": "Biomed Res. Int.",
"key": "ref_14",
"volume": "2022",
"year": "2022"
},
{
"key": "ref_15",
"unstructured": "Abbas, A.K., Lichtman, A.H., and Pillai, S. (2018). Cytokines. Cellular and Molecular Immunology, Elsevier. [9th ed]."
},
{
"DOI": "10.1038/s41419-020-2636-4",
"article-title": "The inhibition of IL-2/IL-2R gives rise to CD8+ T cell and lymphocyte decrease through JAK1-STAT5 in critical patients with COVID-19 pneumonia",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "429",
"journal-title": "Cell Death Dis.",
"key": "ref_16",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2024.1444643",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Niu, C., Liang, T., Chen, Y., Zhu, S., Zhou, L., Chen, N., Qian, L., Wang, Y., Li, M., and Zhou, X. (2024). SARS-CoV-2 spike protein induces the cytokine release syndrome by stimulating T cells to produce more IL-2. Front. Immunol., 15."
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19",
"author": "Moore",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Science",
"key": "ref_18",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2022.1033674",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Wang, X., Tang, G., Liu, Y., Zhang, L., Chen, B., Han, Y., Fu, Z., Wang, L., Hu, G., and Ma, Q. (2022). The role of IL-6 in coronavirus, especially in COVID-19. Front. Pharmacol., 13."
},
{
"DOI": "10.1002/jmv.26085",
"article-title": "Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "35",
"journal-title": "J. Med. Virol.",
"key": "ref_20",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s10875-020-00899-z",
"article-title": "Circulating Levels of Interleukin-6 and Interleukin-10, But Not Tumor Necrosis Factor-Alpha, as Potential Biomarkers of Severity and Mortality for COVID-19: Systematic Review with Meta-analysis",
"author": "Udomsinprasert",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "J. Clin. Immunol.",
"key": "ref_21",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2141",
"article-title": "Interleukin-6 in Covid-19: A systematic review and meta-analysis",
"author": "Coomes",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Rev. Med. Virol.",
"key": "ref_22",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1186/s12967-020-02571-x",
"article-title": "Serum interleukin-6 is an indicator for severity in 901 patients with SARS-CoV-2 infection: A cohort study",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "J. Transl. Med.",
"key": "ref_23",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.imbio.2022.152236",
"article-title": "Deciphering the balance of IL-6/IL-10 cytokines in severe to critical COVID-19 patients",
"author": "Azaiz",
"doi-asserted-by": "crossref",
"first-page": "152236",
"journal-title": "Immunobiology",
"key": "ref_24",
"volume": "227",
"year": "2022"
},
{
"DOI": "10.1016/j.cytogfr.2020.05.009",
"article-title": "IL-6: Relevance for immunopathology of SARS-CoV-2",
"author": "Gubernatorova",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Cytokine Growth Factor Rev.",
"key": "ref_25",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.20944/preprints202406.0409.v1",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Di Spigna, G., Covelli, B., Vargas, M., Di Caprio, R., Rubino, V., Iacovazzo, C., Napolitano, F., Servillo, G., and Postiglione, L. (2024). The Behaviour of IL-6 and Its Soluble Receptor Complex during Different Waves of the COVID-19 Pandemic. Life, 14."
},
{
"DOI": "10.3389/fimmu.2022.795315",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Zizzo, G., Tamburello, A., Castelnovo, L., Laria, A., Mumoli, N., Faggioli, P.M., Stefani, I., and Mazzone, A. (2022). Immunotherapy of COVID-19: Inside and Beyond IL-6 Signalling. Front. Immunol., 13."
},
{
"DOI": "10.52601/bpr.2021.200024",
"article-title": "What do we know about IL-6 in COVID-19 so far?",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Biophys. Rep.",
"key": "ref_28",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3390/ijms252111411",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Paranga, T.G., Mitu, I., Pavel-Tanasa, M., Rosu, M.F., Miftode, I.L., Constantinescu, D., Obreja, M., Plesca, C.E., and Miftode, E. (2024). Cytokine Storm in COVID-19: Exploring IL-6 Signaling and Cytokine-Microbiome Interactions as Emerging Therapeutic Approaches. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.1016/j.intimp.2022.109005",
"article-title": "Roles of Interleukin-6-mediated immunometabolic reprogramming in COVID-19 and other viral infection-associated diseases",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "109005",
"journal-title": "Int. Immunopharmacol.",
"key": "ref_30",
"volume": "110",
"year": "2022"
},
{
"DOI": "10.1016/j.autrev.2020.102537",
"article-title": "The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease",
"author": "McGonagle",
"doi-asserted-by": "crossref",
"first-page": "102537",
"journal-title": "Autoimmun. Rev.",
"key": "ref_31",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.3109/15476910903453296",
"article-title": "An overview of IL-7 biology and its use in immunotherapy",
"author": "ElKassar",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J. Immunotoxicol.",
"key": "ref_32",
"volume": "7",
"year": "2010"
},
{
"DOI": "10.1038/s41590-019-0479-x",
"article-title": "Flip the coin: IL-7 and IL-7R in health and disease",
"author": "Barata",
"doi-asserted-by": "crossref",
"first-page": "1584",
"journal-title": "Nat. Immunol.",
"key": "ref_33",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.3390/ijms232214146",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Korobova, Z.R., Arsentieva, N.A., Liubimova, N.E., Batsunov, O.K., Dedkov, V.G., Gladkikh, A.S., Sharova, A.A., Adish, Z., Chernykh, E.I., and Kaschenko, V.A. (2022). Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1001/jamanetworkopen.2020.16485",
"article-title": "Association of Interleukin 7 Immunotherapy with Lymphocyte Counts Among Patients with Severe Coronavirus Disease 2019 (COVID-19)",
"author": "Laterre",
"doi-asserted-by": "crossref",
"first-page": "e2016485",
"journal-title": "JAMA Netw. Open",
"key": "ref_35",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1172/jci.insight.189150",
"article-title": "A randomized, double-blind, placebo-controlled trial of IL-7 in critically ill patients with COVID-19",
"author": "Francois",
"doi-asserted-by": "crossref",
"first-page": "e189150",
"journal-title": "JCI Insight",
"key": "ref_36",
"volume": "10",
"year": "2025"
},
{
"article-title": "Interleukin-7 Reverses Lymphopenia and Improves T-Cell Function in Coronavirus Disease 2019 Patient with Inborn Error of Toll-Like Receptor 3: A Case Report",
"author": "Mazer",
"first-page": "e0500",
"journal-title": "Crit. Care Explor.",
"key": "ref_37",
"volume": "3",
"year": "2021"
},
{
"DOI": "10.3390/v17111416",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Siedlecka, D., Bielawska, L., Ludziejewska, A., Baszczuk, A., and Wysocka, E. (2025). IL-2 and IL-7 Contribution to Immune Response: Effects of Vaccination Against COVID-19 in Adults. Viruses, 17."
},
{
"DOI": "10.3389/fimmu.2021.737406",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Bekele, Y., Sui, Y., and Berzofsky, J.A. (2021). IL-7 in SARS-CoV-2 Infection and as a Potential Vaccine Adjuvant. Front. Immunol., 12."
},
{
"DOI": "10.1038/s41467-023-39815-5",
"article-title": "IL-9 aggravates SARS-CoV-2 infection and exacerbates associated airway inflammation",
"author": "Sadhu",
"doi-asserted-by": "crossref",
"first-page": "4060",
"journal-title": "Nat. Commun.",
"key": "ref_40",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1186/s12865-024-00641-z",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Calvo-Alvarez, E., D’Alessandro, S., Zanotta, N., Basilico, N., Parapini, S., Signorini, L., Perego, F., Maina, K.K., Ferrante, P., and Modenese, A. (2024). Multiplex array analysis of circulating cytokines and chemokines in COVID-19 patients during the first wave of the SARS-CoV-2 pandemic in Milan, Italy. BMC Immunol., 25."
},
{
"DOI": "10.1016/j.cyto.2020.155323",
"article-title": "Cytokine profile and disease severity in patients with COVID-19",
"author": "Ghazavi",
"doi-asserted-by": "crossref",
"first-page": "155323",
"journal-title": "Cytokine",
"key": "ref_42",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1128/mbio.00905-24",
"article-title": "Helminth alleviates COVID-19-related cytokine storm in an IL-9-dependent way",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "e0090524",
"journal-title": "mBio",
"key": "ref_43",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1128/mbio.01229-24",
"article-title": "IL-9 plays a critical role in helminth-induced protection against COVID-19-related cytokine storms",
"author": "Xiang",
"doi-asserted-by": "crossref",
"first-page": "e0122924",
"journal-title": "mBio",
"key": "ref_44",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.33448/rsd-v14i3.48459",
"article-title": "Anti-inflammatory interleukins (IL-4 and IL-10) and their relationship with the severity of COVID-19: A systematic review",
"author": "Santos",
"doi-asserted-by": "crossref",
"first-page": "e4214348459",
"journal-title": "Res. Soc. Dev.",
"key": "ref_45",
"volume": "14",
"year": "2025"
},
{
"DOI": "10.1016/j.heliyon.2021.e06155",
"article-title": "IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression",
"author": "Dhar",
"doi-asserted-by": "crossref",
"first-page": "e06155",
"journal-title": "Heliyon",
"key": "ref_46",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"article-title": "Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "1123",
"journal-title": "Emerg. Microbes Infect.",
"key": "ref_47",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1089/jir.2023.0075",
"article-title": "Characterization of IL-10 Family of Cytokines in Acute and Convalescent COVID-19 Individuals",
"author": "Rajamanickam",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "J. Interferon Cytokine Res.",
"key": "ref_48",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1186/s40246-023-00468-6",
"article-title": "Association between interleukin-10 gene polymorphisms (rs1800871, rs1800872, and rs1800896) and severity of infection in different SARS-CoV-2 variants",
"author": "Abbood",
"doi-asserted-by": "crossref",
"first-page": "19",
"journal-title": "Hum. Genom.",
"key": "ref_49",
"volume": "17",
"year": "2023"
},
{
"DOI": "10.1084/jem.20190418",
"article-title": "Biology and therapeutic potential of interleukin-10",
"author": "Saraiva",
"doi-asserted-by": "crossref",
"first-page": "e20190418",
"journal-title": "J. Exp. Med.",
"key": "ref_50",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.1002/art.22055",
"article-title": "Dysregulation of interleukin-10-dependent gene expression in rheumatoid arthritis synovial macrophages",
"author": "Antoniv",
"doi-asserted-by": "crossref",
"first-page": "2711",
"journal-title": "Arthritis Rheum.",
"key": "ref_51",
"volume": "54",
"year": "2006"
},
{
"DOI": "10.1084/jem.20021820",
"article-title": "Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis",
"author": "Ji",
"doi-asserted-by": "crossref",
"first-page": "1573",
"journal-title": "J. Exp. Med.",
"key": "ref_52",
"volume": "197",
"year": "2003"
},
{
"DOI": "10.3389/fimmu.2021.677008",
"doi-asserted-by": "crossref",
"key": "ref_53",
"unstructured": "Islam, H., Chamberlain, T.C., Mui, A.L., and Little, J.P. (2021). Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action?. Front. Immunol., 12."
},
{
"DOI": "10.3389/fimmu.2022.984098",
"doi-asserted-by": "crossref",
"key": "ref_54",
"unstructured": "Najafi-Fard, S., Petruccioli, E., Farroni, C., Petrone, L., Vanini, V., Cuzzi, G., Salmi, A., Altera, A.M.G., Navarra, A., and Alonzi, T. (2022). Evaluation of the immunomodulatory effects of interleukin-10 on peripheral blood immune cells of COVID-19 patients: Implication for COVID-19 therapy. Front. Immunol., 13."
},
{
"DOI": "10.1080/21505594.2023.2190650",
"article-title": "An important call: Suggestion of using IL-10 as therapeutic agent for COVID-19 with ARDS and other complications",
"author": "Shih",
"doi-asserted-by": "crossref",
"first-page": "2190650",
"journal-title": "Virulence",
"key": "ref_55",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1007/s10787-022-00992-2",
"article-title": "COVID-19 and the role of cytokines in this disease",
"author": "Hasanvand",
"doi-asserted-by": "crossref",
"first-page": "789",
"journal-title": "Inflammopharmacology",
"key": "ref_56",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0274841",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Sharif-Askari, F.S., Sharif-Askari, N.S., Hafezi, S., Mdkhana, B., Alsayed, H.A.H., Ansari, A.W., Mahboub, B., Zakeri, A.M., Temsah, M.H., and Zahir, W. (2022). Interleukin-17, a salivary biomarker for COVID-19 severity. PLoS ONE, 17."
},
{
"DOI": "10.1016/j.immuni.2019.03.021",
"article-title": "The IL-17 Family of Cytokines in Health and Disease",
"author": "McGeachy",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "Immunity",
"key": "ref_58",
"volume": "50",
"year": "2019"
},
{
"DOI": "10.1084/jem.20200652",
"article-title": "Targeting potential drivers of COVID-19: Neutrophil extracellular traps",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "e20200652",
"journal-title": "J. Exp. Med.",
"key": "ref_59",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.3390/cells10061550",
"doi-asserted-by": "crossref",
"key": "ref_60",
"unstructured": "Martonik, D., Parfieniuk-Kowerda, A., Rogalska, M., and Flisiak, R. (2021). The Role of Th17 Response in COVID-19. Cells, 10."
},
{
"DOI": "10.1084/jem.20092121",
"article-title": "Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent",
"author": "Wilson",
"doi-asserted-by": "crossref",
"first-page": "535",
"journal-title": "J. Exp. Med.",
"key": "ref_61",
"volume": "207",
"year": "2010"
},
{
"DOI": "10.1371/journal.ppat.1005973",
"doi-asserted-by": "crossref",
"key": "ref_62",
"unstructured": "Slaats, J., Ten Oever, J., van de Veerdonk, F.L., and Netea, M.G. (2016). IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog., 12."
},
{
"DOI": "10.3389/fimmu.2018.00763",
"doi-asserted-by": "crossref",
"key": "ref_63",
"unstructured": "Nakanishi, K. (2018). Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front. Immunol., 9."
},
{
"DOI": "10.4049/jimmunol.1103685",
"article-title": "IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures",
"author": "Blom",
"doi-asserted-by": "crossref",
"first-page": "4331",
"journal-title": "J. Immunol.",
"key": "ref_64",
"volume": "189",
"year": "2012"
},
{
"DOI": "10.1016/j.cyto.2020.155302",
"article-title": "Prognostic value of interleukin-18 and its association with other inflammatory markers and disease severity in COVID-19",
"author": "Gulbahar",
"doi-asserted-by": "crossref",
"first-page": "155302",
"journal-title": "Cytokine",
"key": "ref_65",
"volume": "137",
"year": "2021"
},
{
"DOI": "10.1007/s10238-022-00943-9",
"article-title": "Interleukin 18 and IL-18 BP response to Sars-CoV-2 virus infection",
"author": "Marino",
"doi-asserted-by": "crossref",
"first-page": "1243",
"journal-title": "Clin. Exp. Med.",
"key": "ref_66",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1017/S0950268821002636",
"article-title": "Interleukin-18 and COVID-19",
"author": "Schooling",
"doi-asserted-by": "crossref",
"first-page": "e14",
"journal-title": "Epidemiol. Infect.",
"key": "ref_67",
"volume": "150",
"year": "2021"
},
{
"DOI": "10.1084/jem.20201707",
"article-title": "Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients",
"author": "Rodrigues",
"doi-asserted-by": "crossref",
"first-page": "e20201707",
"journal-title": "J. Exp. Med.",
"key": "ref_68",
"volume": "218",
"year": "2021"
},
{
"DOI": "10.1111/all.14907",
"article-title": "Caspases and therapeutic potential of caspase inhibitors in moderate-severe SARS-CoV-2 infection and long COVID",
"author": "Plassmeyer",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "Allergy",
"key": "ref_69",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.15252/emmm.202114150",
"article-title": "Long-lived macrophage reprogramming drives spike protein-mediated inflammasome activation in COVID-19",
"author": "Theobald",
"doi-asserted-by": "crossref",
"first-page": "e14150",
"journal-title": "EMBO Mol. Med.",
"key": "ref_70",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.21203/rs.3.rs-153628/v1",
"doi-asserted-by": "crossref",
"key": "ref_71",
"unstructured": "Junqueira, C., Crespo, Â., Ranjbar, S., Ingber, J., Parry, B., Ravid, S., de Lacerda, L.B., Lewandrowski, M., Clark, S., and Ho, F. (2021). SARS-CoV-2 infects blood monocytes to activate NLRP3 and AIM2 inflammasomes, pyroptosis and cytokine release. medRxiv."
},
{
"DOI": "10.1038/s41392-023-01368-w",
"article-title": "SARS-CoV-2 spike protein induces IL-18-mediated cardiopulmonary inflammation via reduced mitophagy",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_72",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1172/jci.insight.148694",
"article-title": "Natural mucosal barriers and COVID-19 in children",
"author": "Pierce",
"doi-asserted-by": "crossref",
"first-page": "e148694",
"journal-title": "JCI Insight",
"key": "ref_73",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2019.01091",
"doi-asserted-by": "crossref",
"key": "ref_74",
"unstructured": "Williams, M.A., O’Callaghan, A., and Corr, S.C. (2019). IL-33 and IL-18 in Inflammatory Bowel Disease Etiology and Microbial Interactions. Front. Immunol., 10."
},
{
"DOI": "10.1016/j.medmic.2020.100023",
"article-title": "Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18",
"author": "Tao",
"doi-asserted-by": "crossref",
"first-page": "100023",
"journal-title": "Med. Microecol.",
"key": "ref_75",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/iid3.476",
"article-title": "Sustained expression of inflammatory monocytes and activated T cells in COVID-19 patients and recovered convalescent plasma donors",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "1279",
"journal-title": "Immun. Inflamm. Dis.",
"key": "ref_76",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2015.08.001",
"article-title": "IL-27-induced modulation of autoimmunity and its therapeutic potential",
"author": "Meka",
"doi-asserted-by": "crossref",
"first-page": "1131",
"journal-title": "Autoimmun. Rev.",
"key": "ref_77",
"volume": "14",
"year": "2015"
},
{
"DOI": "10.1007/s00705-023-05792-9",
"article-title": "Antiviral response and immunopathogenesis of interleukin 27 in COVID-19",
"doi-asserted-by": "crossref",
"first-page": "178",
"journal-title": "Arch. Virol.",
"key": "ref_78",
"volume": "168",
"year": "2023"
},
{
"DOI": "10.1186/s12985-025-02668-z",
"article-title": "Impact of single nucleotide polymorphism of IL-27P28 rs153109 and IFITM3 rs12252 on susceptibility and severity of COVID-19 in Egyptian patients: A case control study",
"author": "Hamdy",
"doi-asserted-by": "crossref",
"first-page": "66",
"journal-title": "Virol. J.",
"key": "ref_79",
"volume": "22",
"year": "2025"
},
{
"DOI": "10.20944/preprints202405.1973.v1",
"doi-asserted-by": "crossref",
"key": "ref_80",
"unstructured": "Korobova, Z.R., Arsentieva, N.A., Santoni, A., and Totolian, A.A. (2024). Role of IL-27 in COVID-19: A Thin Line between Protection and Disease Promotion. Int. J. Mol. Sci., 25."
},
{
"DOI": "10.5811/westjem.2022.3.55325",
"article-title": "Multisystem Inflammatory Syndrome in Children",
"author": "Waseem",
"doi-asserted-by": "crossref",
"first-page": "505",
"journal-title": "West. J. Emerg. Med.",
"key": "ref_81",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2022.992022",
"doi-asserted-by": "crossref",
"key": "ref_82",
"unstructured": "Spracklen, T.F., Mendelsohn, S.C., Butters, C., Facey-Thomas, H., Stander, R., Abrahams, D., Erasmus, M., Baguma, R., Day, J., and Scott, C. (2022). IL27 gene expression distinguishes multisystem inflammatory syndrome in children from febrile illness in a South African cohort. Front. Immunol., 13."
},
{
"article-title": "IL-33-mediated IL-13 secretion by ST2+ Tregs controls inflammation after lung injury",
"author": "Liu",
"first-page": "e123919",
"journal-title": "JCI Insight",
"key": "ref_83",
"volume": "4",
"year": "2019"
},
{
"DOI": "10.1371/journal.pmed.1002365",
"doi-asserted-by": "crossref",
"key": "ref_84",
"unstructured": "Xu, J., Guardado, J., Hoffman, R., Xu, H., Namas, R., Vodovotz, Y., Xu, L., Ramadan, M., Brown, J., and Turnquist, H.R. (2017). IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med., 14."
},
{
"DOI": "10.1186/s12931-020-01511-z",
"article-title": "Inflammatory phenotyping predicts clinical outcome in COVID-19",
"author": "Burke",
"doi-asserted-by": "crossref",
"first-page": "245",
"journal-title": "Respir. Res.",
"key": "ref_85",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3390/v13122415",
"doi-asserted-by": "crossref",
"key": "ref_86",
"unstructured": "Fonseca, W., Asai, N., Yagi, K., Malinczak, C.A., Savickas, G., Johnson, C.C., Murray, S., Zoratti, E.M., Lukacs, N.W., and Li, J. (2021). COVID-19 Modulates Inflammatory and Renal Markers That May Predict Hospital Outcomes among African American Males. Viruses, 13."
},
{
"DOI": "10.3390/ijms232113656",
"doi-asserted-by": "crossref",
"key": "ref_87",
"unstructured": "Gao, Y., Cai, L., Li, L., Zhang, Y., Li, J., Luo, C., Wang, Y., and Tao, L. (2022). Emerging Effects of IL-33 on COVID-19. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1038/s41467-021-22449-w",
"article-title": "IL-33 expression in response to SARS-CoV-2 correlates with seropositivity in COVID-19 convalescent individuals",
"author": "Stanczak",
"doi-asserted-by": "crossref",
"first-page": "2133",
"journal-title": "Nat. Commun.",
"key": "ref_88",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2025.1718240",
"doi-asserted-by": "crossref",
"key": "ref_89",
"unstructured": "Retnakumar, S.V., Singh, S.C., Chauvin, C., and Bayry, J. (2025). IL-33 and IL-3 synergistically induce CD25 expression on human basophils without functional IL-2 signaling: A potential marker of severe COVID-19. Front. Immunol., 16."
},
{
"DOI": "10.3389/fimmu.2021.662266",
"article-title": "The Role of IL-36 in Infectious Diseases: Potential Target for COVID-19?",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "662266",
"journal-title": "Front. Immunol.",
"key": "ref_90",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2",
"author": "Monteil",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "Cell",
"key": "ref_91",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.4081/1184",
"article-title": "ACE2 and AT4R are present in diseased human blood vessels",
"author": "Zulli",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Eur. J. Histochem.",
"key": "ref_92",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0222969",
"doi-asserted-by": "crossref",
"key": "ref_93",
"unstructured": "Mercurio, L., Failla, C.M., Capriotti, L., Scarponi, C., Facchiano, F., Morelli, M., Rossi, S., Pagnanelli, G., Albanesi, C., and Cavani, A. (2020). Interleukin (IL)-17/IL-36 axis participates to the crosstalk between endothelial cells and keratinocytes during inflammatory skin responses. PLoS ONE, 15."
},
{
"DOI": "10.1016/j.cyto.2019.154798",
"article-title": "Increased expression of interleukin 36 in chronic rhinosinusitis and its contribution to chemokine secretion and increased epithelial permeability",
"author": "Joo",
"doi-asserted-by": "crossref",
"first-page": "154798",
"journal-title": "Cytokine",
"key": "ref_94",
"volume": "125",
"year": "2020"
},
{
"key": "ref_95",
"unstructured": "National Center for Immunization and Respiratory Diseases (U.S.) (2023, September 01). Division of Viral Diseases. SARS-CoV-2 Variant Classifications and Definitions, Available online: https://stacks.cdc.gov/view/cdc/133705."
},
{
"DOI": "10.1155/2023/1879554",
"article-title": "COVID-19: An Overview of SARS-CoV-2 Variants-The Current Vaccines and Drug Development",
"author": "Bostanghadiri",
"doi-asserted-by": "crossref",
"first-page": "1879554",
"journal-title": "Biomed Res. Int.",
"key": "ref_96",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.20944/preprints202106.0060.v1",
"doi-asserted-by": "crossref",
"key": "ref_97",
"unstructured": "Raman, R., Patel, K.J., and Ranjan, K. (2021). COVID-19: Unmasking Emerging SARS-CoV-2 Variants, Vaccines and Therapeutic Strategies. Biomolecules, 11."
},
{
"DOI": "10.1007/s44411-024-00010-7",
"article-title": "Comparative study of cytokine profiles in SARS-CoV-2 Delta and Omicron variants",
"author": "Krivosova",
"doi-asserted-by": "crossref",
"first-page": "286",
"journal-title": "Bratisl. Med. J.",
"key": "ref_98",
"volume": "126",
"year": "2025"
},
{
"DOI": "10.1038/s41598-024-76401-1",
"article-title": "SARS-CoV-2 variants induce increased inflammatory gene expression but reduced interferon responses and heme synthesis as compared with wild type strains",
"author": "Merchant",
"doi-asserted-by": "crossref",
"first-page": "25734",
"journal-title": "Sci. Rep.",
"key": "ref_99",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1038/s41598-022-21690-7",
"article-title": "A comparative study of spike protein of SARS-CoV-2 and its variant Omicron (B.1.1.529) on some immune characteristics",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "17058",
"journal-title": "Sci. Rep.",
"key": "ref_100",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1128/spectrum.01256-23",
"article-title": "Analysis of SARS-CoV-2 isolates, namely the Wuhan strain, Delta variant, and Omicron variant, identifies differential immune profiles",
"author": "Shahbaz",
"doi-asserted-by": "crossref",
"first-page": "e0125623",
"journal-title": "Microbiol. Spectr.",
"key": "ref_101",
"volume": "11",
"year": "2023"
},
{
"article-title": "A comprehensive analysis of immune characteristics and clinical prognosis in Asian COVID-19 patients infected with SARS-CoV-2 Omicron strain XBB sub-variants: A retrospective study of 450 cases",
"author": "Li",
"first-page": "1",
"journal-title": "Arch. Med. Sci.",
"key": "ref_102",
"volume": "20",
"year": "2024"
},
{
"DOI": "10.3390/biomedicines13092128",
"doi-asserted-by": "crossref",
"key": "ref_103",
"unstructured": "Barreto, M.A., Cruz, A.M.S., Volle, D.M., Júnior, W.D.D.C., Costa, I.B., Nunes, J.A.L., de Sousa, A.C.P., Lima, I.K.M., Nogami, P.Y., and Borges, I.R. (2025). Clinical Manifestations and Cytokine Profiles of the Th1, Th2, and Th17 Response Associated with SARS-CoV-2 Omicron Subvariants. Biomedicines, 13."
},
{
"DOI": "10.1038/s41564-023-01588-4",
"article-title": "Evolution of enhanced innate immune suppression by SARS-CoV-2 Omicron subvariants",
"author": "Reuschl",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Nat. Microbiol.",
"key": "ref_104",
"volume": "9",
"year": "2024"
},
{
"DOI": "10.1186/s12879-024-09652-y",
"doi-asserted-by": "crossref",
"key": "ref_105",
"unstructured": "Liu, Y., Guo, Y., Zhan, H., Liu, X., Li, X., Cui, J., Li, H., Feng, S., Cheng, L., and Li, X. (2024). Immune and inflammation features of severe and critical Omicron infected patients during Omicron wave in China. BMC Infect. Dis., 24."
},
{
"DOI": "10.5812/jjm-159675",
"article-title": "Effect of SARS-CoV-2 variants (Alpha, Beta, Delta, Omicron) on inflammatory parameters",
"author": "Irvem",
"doi-asserted-by": "crossref",
"first-page": "e159675",
"journal-title": "Jundishapur J. Microbiol.",
"key": "ref_106",
"volume": "18",
"year": "2025"
},
{
"DOI": "10.1002/JLB.5MR0821-464R",
"article-title": "T follicular helper cells in the humoral immune response to SARS-CoV-2 infection and vaccination",
"author": "Koutsakos",
"doi-asserted-by": "crossref",
"first-page": "355",
"journal-title": "J. Leukoc. Biol.",
"key": "ref_107",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.3389/fimmu.2024.1377014",
"doi-asserted-by": "crossref",
"key": "ref_108",
"unstructured": "Kawasuji, H., Morinaga, Y., Nagaoka, K., Tani, H., Yoshida, Y., Yamada, H., Takegoshi, Y., Kaneda, M., Murai, Y., and Kimoto, K. (2024). High interleukin-6 levels induced by COVID-19 pneumonia correlate with increased circulating follicular helper T cell frequency and strong neutralization antibody response in the acute phase of Omicron breakthrough infection. Front. Immunol., 15."
},
{
"DOI": "10.1089/jir.2022.0185",
"article-title": "The Role of Inflammatory Cytokines (Interleukin-1 and Interleukin-6) as a Potential Biomarker in the Different Stages of COVID-19 (Mild, Severe, and Critical)",
"author": "Jami",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "J. Interferon Cytokine Res.",
"key": "ref_109",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.1007/s00011-022-01596-w",
"article-title": "The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19",
"author": "Makaremi",
"doi-asserted-by": "crossref",
"first-page": "923",
"journal-title": "Inflamm. Res.",
"key": "ref_110",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.3390/jcm9124021",
"doi-asserted-by": "crossref",
"key": "ref_111",
"unstructured": "Lariccia, V., Magi, S., Serfilippi, T., Toujani, M., Gratteri, S., and Amoroso, S. (2020). Challenges and Opportunities from Targeting Inflammatory Responses to SARS-CoV-2 Infection: A Narrative Review. J. Clin. Med., 9."
},
{
"article-title": "Interleukin-1 blocking agents for treating COVID-19",
"author": "Davidson",
"first-page": "CD015308",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_112",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1038/s41598-020-78492-y",
"article-title": "Blockage of interleukin-1β with canakinumab in patients with Covid-19",
"author": "Landi",
"doi-asserted-by": "crossref",
"first-page": "21775",
"journal-title": "Sci. Rep.",
"key": "ref_113",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41598-024-81028-3",
"article-title": "Prognostic significance of serum interleukin-6 in severe/critical COVID-19 patients treated with tocilizumab: A detailed observational study analysis",
"author": "Liang",
"doi-asserted-by": "crossref",
"first-page": "29634",
"journal-title": "Sci. Rep.",
"key": "ref_114",
"volume": "14",
"year": "2024"
},
{
"key": "ref_115",
"unstructured": "World Health Organization WHO Prequalifies First Monoclonal Antibody—Tocilizumab—To Treat COVID-19, World Health Organization. Available online: https://www.who.int/news/item/11-02-2022-who-prequalifies-first-monoclonal-antibody---tocilizumab-to-treat-covid-19."
},
{
"article-title": "Interleukin-6 blocking agents for treating COVID-19: A living systematic review",
"author": "Ghosn",
"first-page": "CD013881",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_116",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.3389/fphar.2020.615972",
"doi-asserted-by": "crossref",
"key": "ref_117",
"unstructured": "Han, Q., Guo, M., Zheng, Y., Zhang, Y., De, Y., Xu, C., Zhang, L., Sun, R., Lv, Y., and Liang, Y. (2020). Current Evidence of Interleukin-6 Signaling Inhibitors in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol., 11."
},
{
"DOI": "10.1016/j.ijsu.2022.106593",
"article-title": "WHO prequalified tocilizumab and vaccine boosters against COVID-19",
"author": "Rahimi",
"doi-asserted-by": "crossref",
"first-page": "106593",
"journal-title": "Int. J. Surg.",
"key": "ref_118",
"volume": "99",
"year": "2022"
},
{
"DOI": "10.1080/1744666X.2021.1919086",
"article-title": "The role of IL-6 and IL-6 blockade in COVID-19",
"author": "Potere",
"doi-asserted-by": "crossref",
"first-page": "601",
"journal-title": "Expert. Rev. Clin. Immunol.",
"key": "ref_119",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1038/s41598-024-74196-9",
"article-title": "Pharmacodynamic, prognostic, and predictive biomarkers in severe and critical COVID-19 patients treated with sirukumab",
"author": "Thys",
"doi-asserted-by": "crossref",
"first-page": "22981",
"journal-title": "Sci. Rep.",
"key": "ref_120",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2022.951107",
"doi-asserted-by": "crossref",
"key": "ref_121",
"unstructured": "Fang, S., Ju, D., Lin, Y., and Chen, W. (2022). The role of interleukin-22 in lung health and its therapeutic potential for COVID-19. Front. Immunol., 13."
},
{
"DOI": "10.3389/fimmu.2022.769839",
"doi-asserted-by": "crossref",
"key": "ref_122",
"unstructured": "Albayrak, N., Orte Cano, C., Karimi, S., Dogahe, D., Van Praet, A., Godefroid, A., Del Marmol, V., Grimaldi, D., Bondue, B., and Van Vooren, J.P. (2022). Distinct Expression Patterns of Interleukin-22 Receptor 1 on Blood Hematopoietic Cells in SARS-CoV-2 Infection. Front. Immunol., 13."
},
{
"DOI": "10.1038/s42003-021-02176-0",
"article-title": "Enterocytes, fibroblasts and myeloid cells synergize in anti-bacterial and anti-viral pathways with IL22 as the central cytokine",
"author": "Klooster",
"doi-asserted-by": "crossref",
"first-page": "631",
"journal-title": "Commun. Biol.",
"key": "ref_123",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1002/cti2.1224",
"article-title": "Innate lymphoid cell composition associates with COVID-19 disease severity",
"author": "Kokkinou",
"doi-asserted-by": "crossref",
"first-page": "e1224",
"journal-title": "Clin. Transl. Immunol.",
"key": "ref_124",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01160",
"doi-asserted-by": "crossref",
"key": "ref_125",
"unstructured": "Alcorn, J.F. (2020). IL-22 Plays a Critical Role in Maintaining Epithelial Integrity During Pulmonary Infection. Front. Immunol., 11."
},
{
"DOI": "10.1016/j.ajpath.2012.12.007",
"article-title": "IL-22 is essential for lung epithelial repair following influenza infection",
"author": "Pociask",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "Am. J. Pathol.",
"key": "ref_126",
"volume": "182",
"year": "2013"
},
{
"DOI": "10.3389/fimmu.2017.00254",
"doi-asserted-by": "crossref",
"key": "ref_127",
"unstructured": "Bhaumik, S., and Basu, R. (2017). Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response. Front. Immunol., 8."
},
{
"DOI": "10.1016/j.jmii.2020.03.005",
"article-title": "TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "368",
"journal-title": "J. Microbiol. Immunol. Infect.",
"key": "ref_128",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.05.072",
"article-title": "Interleukin-17: A potential therapeutic target in COVID-19",
"author": "Mendoza",
"doi-asserted-by": "crossref",
"first-page": "e136",
"journal-title": "J. Infect.",
"key": "ref_129",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1080/23744235.2022.2066171",
"article-title": "Blockade of interleukin seventeen (IL-17A) with secukinumab in hospitalized COVID-19 patients—The BISHOP study",
"author": "Resende",
"doi-asserted-by": "crossref",
"first-page": "591",
"journal-title": "Infect. Dis.",
"key": "ref_130",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1016/j.cyto.2021.155627",
"article-title": "Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study",
"author": "Avdeev",
"doi-asserted-by": "crossref",
"first-page": "155627",
"journal-title": "Cytokine",
"key": "ref_131",
"volume": "146",
"year": "2021"
},
{
"DOI": "10.15252/emmm.202012697",
"article-title": "Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients",
"author": "Stebbing",
"doi-asserted-by": "crossref",
"first-page": "e12697",
"journal-title": "EMBO Mol. Med.",
"key": "ref_132",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.2000554",
"article-title": "A Case for Targeting Th17 Cells and IL-17A in SARS-CoV-2 Infections",
"author": "Orlov",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "J. Immunol.",
"key": "ref_133",
"volume": "205",
"year": "2020"
},
{
"DOI": "10.1186/s12979-024-00444-1",
"article-title": "Kinetics of pro- and anti-inflammatory spike-specific cellular immune responses in long-term care facility residents after COVID-19 mRNA primary and booster vaccination: A prospective longitudinal study in Japan",
"author": "Kakugawa",
"doi-asserted-by": "crossref",
"first-page": "41",
"journal-title": "Immun. Ageing",
"key": "ref_134",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.3389/fimmu.2022.869990",
"doi-asserted-by": "crossref",
"key": "ref_135",
"unstructured": "Hurme, A., Jalkanen, P., Heroum, J., Liedes, O., Vara, S., Melin, M., Teräsjärvi, J., He, Q., Pöysti, S., and Hänninen, A. (2022). Long-Lasting T Cell Responses in BNT162b2 COVID-19 mRNA Vaccinees and COVID-19 Convalescent Patients. Front. Immunol., 13."
},
{
"DOI": "10.1016/j.celrep.2021.109504",
"article-title": "Systemic IL-15, IFN-γ, and IP-10/CXCL10 signature associated with effective immune response to SARS-CoV-2 in BNT162b2 mRNA vaccine recipients",
"author": "Bergamaschi",
"doi-asserted-by": "crossref",
"first-page": "109504",
"journal-title": "Cell Rep.",
"key": "ref_136",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1016/j.ajt.2023.05.025",
"article-title": "The role of interleukin-21 in COVID-19 vaccine-induced B cell-mediated immune responses in patients with kidney disease and kidney transplant recipients",
"author": "Malahe",
"doi-asserted-by": "crossref",
"first-page": "1411",
"journal-title": "Am. J. Transplant.",
"key": "ref_137",
"volume": "23",
"year": "2023"
}
],
"reference-count": 137,
"references-count": 137,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/27/3/1391"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Interleukins in COVID-19 and SARS-CoV-2 Variants: Immunopathogenesis, Therapeutic Perspectives and Vaccine-Induced Immune Responses",
"type": "journal-article",
"update-policy": "https://doi.org/10.3390/mdpi_crossmark_policy",
"volume": "27"
}