Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID‐19: coincidence or underestimated risk factor?
et al., Journal of Internal Medicine, doi:10.1111/joim.13121, Jul 2020
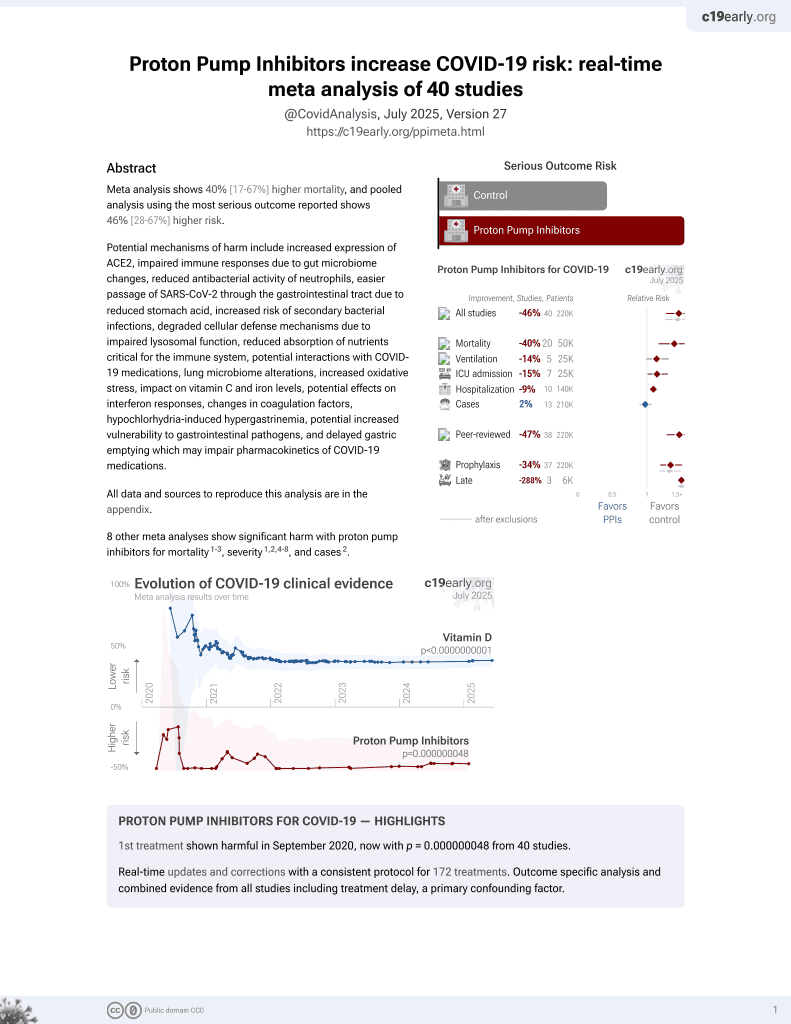
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 152 hospitalized COVID-19 patients showing increased risk of secondary infections, ARDS, and mortality with proton pump inhibitor (PPI) use. Authors hypothesize that reduced gastric acid production from PPIs leads to bacterial overgrowth and microaspiration, increasing the risk of secondary lung infections. PPIs may also have immunomodulatory effects.
|
risk of death, 248.4% higher, RR 3.48, p = 0.02, treatment 12 of 62 (19.4%), control 5 of 90 (5.6%), adjusted per study, multivariable, excluded in exclusion analyses:
unadjusted differences between groups.
|
|
risk of ARDS, 124.3% higher, RR 2.24, p = 0.02, treatment 17 of 62 (27.4%), control 11 of 90 (12.2%), adjusted per study, multivariable, excluded in exclusion analyses:
unadjusted differences between groups.
|
|
secondary infection, 86.0% higher, RR 1.86, p = 0.03, treatment 30 of 62 (48.4%), control 18 of 90 (20.0%), adjusted per study, odds ratio converted to relative risk, multivariable.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Luxenburger et al., 31 Jul 2020, retrospective, Germany, peer-reviewed, 9 authors.
Abstract: Letter to the Editor
doi: 10.1111/joim.13121
Treatment with proton pump inhibitors increases the risk of
secondary infections and ARDS in hospitalized patients with
COVID-19: coincidence or underestimated risk factor?
Dear Sir,
In December 2019, several cases of pneumonia of
unknown origin have been reported in China, and
later, SARS-CoV2 was identified as the causative
pathogen for coronavirus disease 2019 (COVID-19)
[1]. In some cases of COVID-19, the clinical
courses are more severe and may be aggravated
by secondary infections and the development of an
acute respiratory distress syndrome (ARDS) with a
high morbidity and mortality [1]. However, risk
factors for severe clinical courses including
patients’ medication are poorly described. Proton
pump inhibitors (PPI) play an important role in the
treatment of acid-related disorders. As a result of
their high efficacy, PPIs have become one of the
most commonly prescribed agents. However, PPIs
may trigger the development of pneumonia [2] due
to the reduced gastric acid production with subsequent bacterial overgrowth in the upper gastrointestinal tract and microaspiration with following
colonization of the pneumonia [2]. Therefore, we
hypothesized that PPI treatment may also be a
potential risk factor for the development of secondary infections and of ARDS in hospitalized
patients with COVID-19.
In total, 152 patients with confirmed SARS-Cov-2
infection were included in the analysis (Figure S1).
Baseline characteristics are summarized in
Table 1. Sixty-two patients (40.8%) received regular treatment with PPI. Importantly, in 30 patients
(48.4%), no clear reason for the PPI intake was
detectable in the medical records of the patients
and during assessment of patients’ medical history. Forty-eight patients (31.6%) presented with a
secondary infection during hospitalization. In
patients with PPI treatment, 30 of 62 patients
(48.4%) presented with secondary infection compared to 11 of 90 patients (20.0%) without PPI
treatment (P < 0.001, Table 1) indicating that PPI
treatment is a significant risk factor for the
[Correction added on 23 July 2020, after first online publication: The
percentage, (48.4%)“ has been corrected to (27.4%)” in the preceding
sentence.]
development of secondary infections in patients
with SARS-CoV-2 infection. After adjusting for
other risk factors, especially for other predisposing
comorbidities, PPI treatment remained a significant predictive factor for development of secondary
infection (OR 2.37 [01.08–5.22], P = 0.032,
Table S2). Moreover, gastroesophageal reflux disease also emerged as a significant independent
predictive factor of secondary infection (OR 6.40
[1.50–35.51]; P = 0.034) underlining the role of
microaspiration in the pathogenesis of secondary
infection in these patients.
Further, PPI-treated patients developed ARDS in
17 of 62 patients (27.4%) compared to 11 of 90
patients (12.2%) without PPI treatment (P = 0.020,
Table 1). However, development of ARDS was
strongly associated with the presence of a secondary infection as only two patients (1.9%) without a secondary infection developed ARDS
compared to 26 patients (54.2%) with confirmed
secondary infection (P < 0.001). In summary, PPIs
have an indirect effect on ARDS development by
triggering secondary infection. In accordance with
the increased risk of a secondary infection and
consecutive development of ARDS, PPI-treated
patients showed a significantly higher index mortality (19.4% vs. 5.6%, P =..
DOI record:
{
"DOI": "10.1111/joim.13121",
"ISSN": [
"0954-6820",
"1365-2796"
],
"URL": "http://dx.doi.org/10.1111/joim.13121",
"alternative-id": [
"10.1111/joim.13121"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-05-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-05-29"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-07-01"
}
],
"author": [
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
},
{
"name": "IMM‐PACT Faculty of Medicine University of Freiburg Freiburg Germany"
}
],
"family": "Luxenburger",
"given": "H.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
}
],
"family": "Sturm",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine III (Interdisciplinary Medical Intensive Care) Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
},
{
"name": "Department of Cardiology and Angiology I Heart Center University of Freiburg Freiburg Germany"
}
],
"family": "Biever",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
}
],
"family": "Rieg",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine III (Interdisciplinary Medical Intensive Care) Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
},
{
"name": "Department of Cardiology and Angiology I Heart Center University of Freiburg Freiburg Germany"
}
],
"family": "Duerschmied",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
}
],
"family": "Schultheiss",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
}
],
"family": "Neumann‐Haefelin",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
}
],
"family": "Thimme",
"given": "R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8782-8729",
"affiliation": [
{
"name": "From the Department of Medicine II Faculty of Medicine University Medical Center ‐ University of Freiburg Freiburg Germany"
},
{
"name": "Berta‐Ottenstein‐Programme Faculty of Medicine University of Freiburg Freiburg Germany"
}
],
"authenticated-orcid": false,
"family": "Bettinger",
"given": "D.",
"sequence": "additional"
}
],
"container-title": "Journal of Internal Medicine",
"container-title-short": "J. Intern. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
7,
1
]
],
"date-time": "2020-07-01T09:34:05Z",
"timestamp": 1593596045000
},
"deposited": {
"date-parts": [
[
2023,
9,
3
]
],
"date-time": "2023-09-03T12:27:18Z",
"timestamp": 1693744038000
},
"indexed": {
"date-parts": [
[
2024,
7,
19
]
],
"date-time": "2024-07-19T00:48:14Z",
"timestamp": 1721350094861
},
"is-referenced-by-count": 46,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
7
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
7,
1
]
],
"date-time": "2020-07-01T00:00:00Z",
"timestamp": 1593561600000
}
}
],
"link": [
{
"URL": "https://api.wiley.com/onlinelibrary/tdm/v1/articles/10.1111%2Fjoim.13121",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/joim.13121",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/joim.13121",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/joim.13121",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "121-124",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
7
]
]
},
"published-online": {
"date-parts": [
[
2020,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_2_1"
},
{
"DOI": "10.1503/cmaj.092129",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_3_1"
},
{
"DOI": "10.1371/journal.pone.0128004",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_4_1"
},
{
"DOI": "10.2174/1389200219666171207125351",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_5_1"
},
{
"DOI": "10.1111/j.1365-2710.2008.00907.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_6_1"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "e_1_2_1_4_7_1"
}
],
"reference-count": 6,
"references-count": 6,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/joim.13121"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Treatment with proton pump inhibitors increases the risk of secondary infections and ARDS in hospitalized patients with COVID‐19: coincidence or underestimated risk factor?",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "289"
}