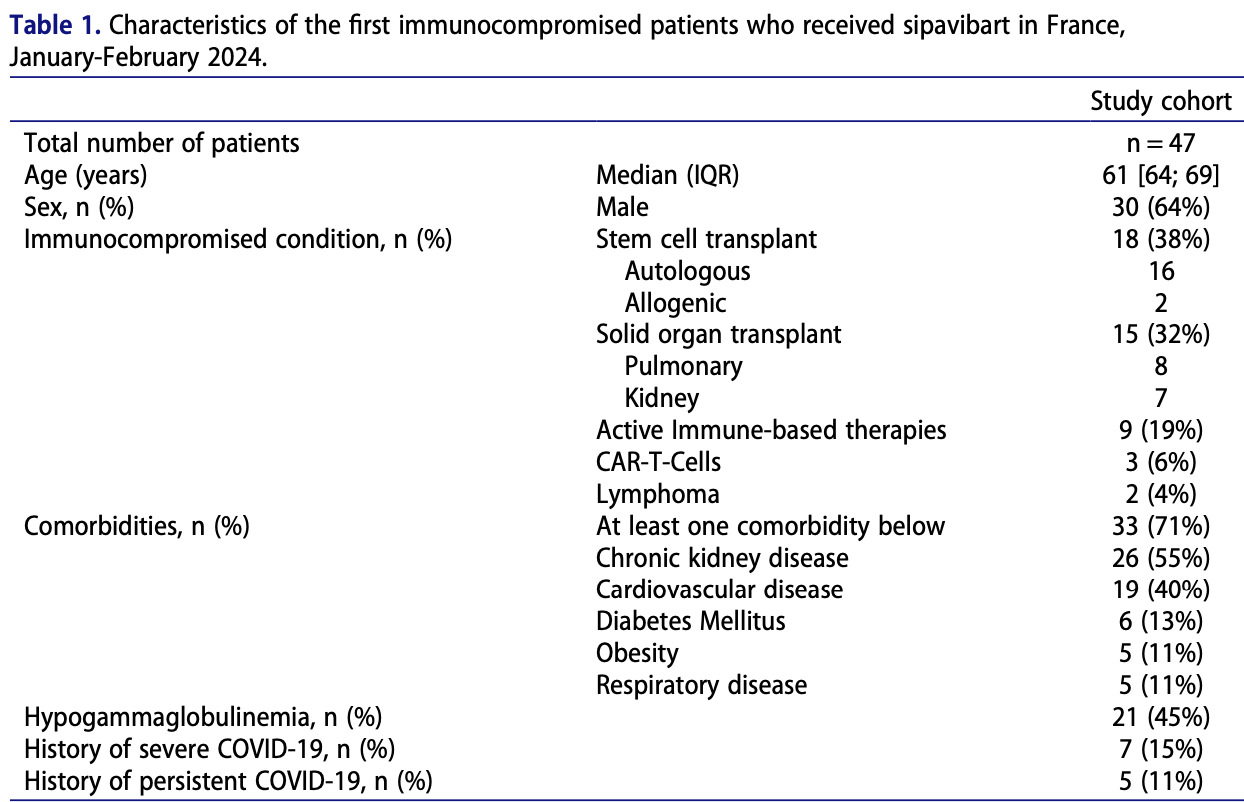
Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France
et al., Human Vaccines & Immunotherapeutics, doi:10.1080/21645515.2024.2387221, Aug 2024
Retrospective 47 immunocompromised patients in France showing no adverse events with sipavibart, an investigational long-acting monoclonal antibody, as COVID-19 pre-exposure prophylaxis. The patients had various immunosuppressive conditions, frequently with hypogammaglobulinemia and other comorbidities.
Loubet et al., 14 Aug 2024, retrospective, France, peer-reviewed, 6 authors.
Contact: paul.loubet@chu-nimes.fr.
Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France
Human Vaccines & Immunotherapeutics, doi:10.1080/21645515.2024.2387221
France was the first country to grant Sipavibart (AZD3152, an investigational long-acting monoclonal antibody) as a COVID-19 pre-exposure prophylaxis treatment in immunocompromised individuals in December 2023. The first patients to receive Sipavibart had different profiles, but they were all highly immunocompromised with frequently associated hypogammaglobulinemia and other chronic conditions. No adverse event was reported.
Disclosure statement Paul Loubet has received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from AstraZeneca, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Sanofi Pasteur.
Contributors' statement PL : Conceptualization, methodology, analysis and original draft; PL, BG, MS, HG, IB, TS: writing -review and editing, All authors read, revised, and approved the final manuscript. He received his M.D., specializing in Infectious and Tropical Diseases, and his Ph.D. from the Université de Paris, with his research on diagnosing lower respiratory infections in adults.
Corresponding author's bio His main areas of interest are respiratory infections in adults from prevention to diagnosis and treatment, respiratory viruses, epidemiology of vaccine-preventable diseases, vaccines, and HIV.
References
Evans, Dube, Lu, Yates, Arnetorp et al., Impact of COVID-19 on immunocompromised populations during the omicron era: insights from the observational population-based INFORM study, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2023.100747
Levin, Ustianowski, Wit, Launay, Avila et al., Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620
Suribhatla, Starkey, Ionescu, Pagliuca, Richter et al., Systematic review and meta-analysis of the clinical effectiveness of tixagevimab/cilgavimab for prophylaxis of COVID-19 in immunocompromised patients, Br J Haematol, doi:10.1111/bjh.18782
DOI record:
{
"DOI": "10.1080/21645515.2024.2387221",
"ISSN": [
"2164-5515",
"2164-554X"
],
"URL": "http://dx.doi.org/10.1080/21645515.2024.2387221",
"alternative-id": [
"10.1080/21645515.2024.2387221"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=khvi20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=khvi20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-07-04"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-07-28"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2024-08-14"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1218-9432",
"affiliation": [
{
"name": "Virulence Bactérienne et Infections Chroniques, University Montpellier, Nîmes, France"
}
],
"authenticated-orcid": false,
"family": "Loubet",
"given": "Paul",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Service des Maladies Infectieuses et Tropicales, CHU Nantes, Nantes, France"
}
],
"family": "Gaborit",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Pneumologie, AP-HP, Hôpital Bichat, Paris, France"
}
],
"family": "Salpin",
"given": "Mathilde",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service d’Hématologie, CHU Poitiers, Poitiers, France"
}
],
"family": "Gardeney",
"given": "Hèlene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service de Néphrologie-Dialyse-Transplantation, CHU Strasbourg, Strasbourg, France"
}
],
"family": "Benotmane",
"given": "Ilies",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Service d’Hématologie, CHU Poitiers, Poitiers, France"
}
],
"family": "Systchenko",
"given": "Thomas",
"sequence": "additional"
}
],
"container-title": "Human Vaccines & Immunotherapeutics",
"container-title-short": "Human Vaccines & Immunotherapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2024,
8,
15
]
],
"date-time": "2024-08-15T06:26:00Z",
"timestamp": 1723703160000
},
"deposited": {
"date-parts": [
[
2024,
8,
15
]
],
"date-time": "2024-08-15T06:26:03Z",
"timestamp": 1723703163000
},
"indexed": {
"date-parts": [
[
2024,
8,
16
]
],
"date-time": "2024-08-16T00:09:20Z",
"timestamp": 1723766960531
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
8,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2024,
12,
31
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
14
]
],
"date-time": "2024-08-14T00:00:00Z",
"timestamp": 1723593600000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/21645515.2024.2387221",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2024,
8,
14
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
14
]
]
},
"published-print": {
"date-parts": [
[
2024,
12,
31
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.lanepe.2023.100747",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_2_1"
},
{
"DOI": "10.1056/NEJMoa2116620",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_1"
},
{
"DOI": "10.1111/bjh.18782",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_1"
}
],
"reference-count": 3,
"references-count": 3,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/21645515.2024.2387221"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Characteristics of the first immunocompromised patients to receive sipavibart as an early access treatment for COVID-19 pre-exposure prophylaxis in France",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "20"
}