Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19: A Randomized Clinical Trial
et al., JAMA, doi:10.1001/jama.2021.3071, Mar 2021
Ivermectin for COVID-19
4th treatment shown to reduce risk in
August 2020, now with p < 0.00000000001 from 106 studies, recognized in 24 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
See also
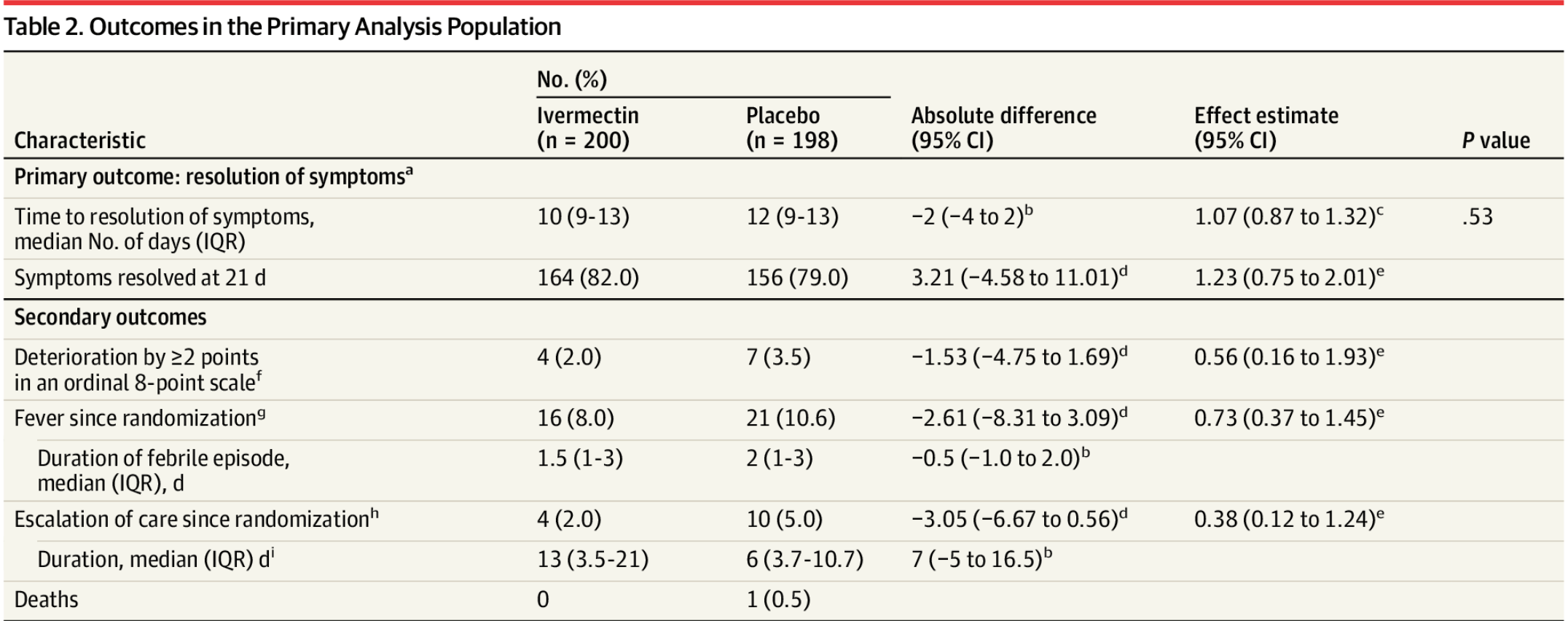
Phone survey based RCT with low risk patients, 200 ivermectin
and 198 control, showing lower mortality, lower disease progression, lower
treatment escalation, and faster resolution of symptoms with treatment,
without reaching statistical significance. Authors find the results of this
trial alone do not support the use of ivermectin. However the effects are all
positive, especially for serious outcomes which are unable to reach
statistical significance with the very small number of events in the low risk
population.
An open letter, signed by >100 physicians, concluding this
study is fatally flawed can be found at jamaletter.com.
With the low risk patient population, there is little room for
improvement with an effective treatment - 59/57% (IVM/control) recovered
within the first 2 days to either "no symptoms" or "not hospitalized and no
limitation of activities"; 73/69% within 5 days. Less than 3% of all patients
ever deteriorated.
The primary outcome was changed mid-trial, it was originally
clinical deterioration, which is more meaningful, and shows greater benefit.
The new outcome of resolution of symptoms includes "not hospitalized and no
limitation of activities" as a negative outcome and is not very meaningful in
terms of assessing how much treatment reduces serious outcomes. Using this
measure could completely invalidate results - for example a treatment that
eliminates all COVID-19 symptoms but has a temporary minor adverse event
could be seen as worse.
Authors state that "preliminary reports of other randomized
trials of ivermectin as treatment for COVID-19 with positive results have not
yet been published in peer-reviewed journals", however there were 8
peer-reviewed RCTs with positive effects published prior to this paper(and 19
total peer-reviewed studies with positive effects).
Authors advised taking ivermectin on an empty stomach, reducing
lung tissue concentration by ~2.5x2.
76 patients were excluded due to control patients receiving
ivermectin. However, there was a similar percentage of adverse events like
diarrhea, nausea, and abdominal pain in both treatment and control groups.
These are potential non-serious side effects of treatment and suggest that it
is possible that many more control patients received some kind of
treatment.
No pre-registered protocol documentation has been found, the
same organization is associated with other COVID trials with extremely high
financial conflicts of interest with this trial, and the official registration
shows a different code to the paper (IVE-PA_CEIP vs. PI-CEP-1390)3.
Ivermectin was widely used in the population and available OTC
at the time of the study. The paper claims that patients were excluded if they
used ivermectin within the last 5 days, however this conflicts with the trial
registration which shows that use of ivermectin within the previous 2 days was
an exclusion criterion. A post-hoc change to 5 days was made on December 16,
20204,5,
which is after enrollment ended (July 15 to November 30, 2020). Ivermectin
may retain efficacy far beyond 2 or 5 days. Note that, with 75% of patients
having symptoms for 4+ days at baseline, the trial registration allows
patients to take ivermectin for a few days after symptoms and then join the
placebo arm two days later6.
The study reports 11.5% blurry vision with ivermectin,
consistent with known side effects. However, the study also reports 11.6%
blurry vision in the placebo group, which is not consistent with expected side
effects of placebo. One possible explanation is that many placebo patients
received ivermectin.
This study reportedly has an ethical issue whereby participants
were told the study drug was "D11AX22"7.
The editor-in-chief of JAMA initially offered to help with this issue, but
later indicated that "JAMA does not review consent forms", however the lead
author reportedly confirmed the issue8-10.
The study protocol specifically allows "the use of other
treatments outside of clinical trials". The paper provides no information on
what other treatments were used, but other treatments were commonly used at
the time. Additionally, the control group did about 5x better than
anticipated for deterioration, also suggesting that the control patients used
some kind of treatment. Patients that enroll in such a study may be more
likely to learn about and use other treatments, especially since they do not
know if they are receiving the study medication.
The study protocol was amended 4 times. Amendments 2-4 are
provided but amendment 1 is missing. Amendment 2 increased the inclusion
criteria to within 7 days of onset, including more later stage patients and
reducing the expected effectiveness. The trial protocol lists “the duration
of supplemental oxygen” as an outcome but the results for this outcome are
missing.
RCTs have a fundamental bias against finding an effect for
interventions that are widely available — patients that believe they
need treatment are more likely to decline participation and take the
intervention11, i.e., RCTs are more likely to enroll low-risk
participants that do not need treatment to recover (this does not apply to
the typical pharmaceutical trial of a new drug that is otherwise
unavailable). This trial was run in a community where ivermectin was
available OTC and very widely known and used.
Grants and/or personal fees, including in some cases during the
conduct of the study, were provided by Sanofi Pasteur, GlaxoSmithKline,
Janssen, Merck, and Gilead. For more details see12.
For other confounding issues see13 and additional
issues can be found in the comments of the article14.
Re-analysis of the raw data has been reported to show a significant positive effect15.
Most data was collected via surveys, without physical
examination. 87% medication adherence. NCT04405843 (history).
This is the 19th of 53 COVID-19 RCTs for ivermectin, which collectively show efficacy with p=0.000000087.
This is the 41st of 106 COVID-19 controlled studies for ivermectin, which collectively show efficacy with p<0.0000000001.
This study is excluded in the after exclusion results of meta-analysis:
strong evidence of patients in the control group self-medicating, ivermectin widely used in the population at that time, and the study drug identity was concealed by using the name D11AX22.
|
risk of death, 66.8% lower, RR 0.33, p = 0.50, treatment 0 of 200 (0.0%), control 1 of 198 (0.5%), NNT 198, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of escalation of care, 60.8% lower, RR 0.39, p = 0.11, treatment 4 of 200 (2.0%), control 10 of 198 (5.1%), NNT 33, odds ratio converted to relative risk.
|
|
risk of escalation of care with post-hoc <12h exclusion, 34.3% lower, RR 0.66, p = 0.52, treatment 4 of 200 (2.0%), control 6 of 198 (3.0%), NNT 97, odds ratio converted to relative risk.
|
|
risk of deterioration by ≥2 points on an 8-point scale, 43.1% lower, RR 0.57, p = 0.37, treatment 4 of 200 (2.0%), control 7 of 198 (3.5%), NNT 65, odds ratio converted to relative risk, primary outcome.
|
|
risk of fever post randomization, 24.8% lower, RR 0.75, p = 0.38, treatment 16 of 200 (8.0%), control 21 of 198 (10.6%), NNT 38, odds ratio converted to relative risk.
|
|
risk of unresolved symptoms at day 21, 15.3% lower, RR 0.85, p = 0.53, treatment 36 of 200 (18.0%), control 42 of 198 (21.2%), NNT 31, inverted to make RR<1 favor treatment, odds ratio converted to relative risk, Cox proportional-hazard model.
|
|
lack of resolution of symptoms, 6.5% lower, HR 0.93, p = 0.53, treatment 200, control 198, inverted to make HR<1 favor treatment, post-hoc primary outcome.
|
|
relative median time to resolution of symptoms, 16.7% better, relative time 0.83, treatment 200, control 198.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
2.
Guzzo et al., Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects, J. Clinical Pharmacology, doi:10.1177/009127002237994.
8.
trialsitenews.com (B), trialsitenews.com/jama-unable-to-confirm-ivermectin-study-consent-form-used-ivermectin/.
9.
trialsitenews.com (C), trialsitenews.com/jama-ivermectin-study-deception-of-study-participants-is-publicly-confirmed/.
10.
francesoir.fr, www.francesoir.fr/societe-sante/la-tromperie-de-letude-jama-sur-livermectine-des-participants-letude-est-confirmee.
11.
Yeh et al., Parachute use to prevent death and major trauma when jumping from aircraft: randomized controlled trial, BMJ, doi:10.1136/bmj.k5094.
López-Medina et al., 4 Mar 2021, Double Blind Randomized Controlled Trial, Colombia, peer-reviewed, median age 37.0, 19 authors, study period 15 July, 2020 - 30 November, 2020, average treatment delay 5.0 days, dosage 300μg/kg days 1-5.
Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19
JAMA, doi:10.1001/jama.2021.3071
IMPORTANCE Ivermectin is widely prescribed as a potential treatment for COVID-19 despite uncertainty about its clinical benefit. OBJECTIVE To determine whether ivermectin is an efficacious treatment for mild COVID-19. DESIGN, SETTING, AND PARTICIPANTS Double-blind, randomized trial conducted at a single site in Cali, Colombia. Potential study participants were identified by simple random sampling from the state's health department electronic database of patients with symptomatic, laboratory-confirmed COVID-19 during the study period. A total of 476 adult patients with mild disease and symptoms for 7 days or fewer (at home or hospitalized) were enrolled between July 15 and November 30, 2020, and followed up through December 21, 2020. INTERVENTION Patients were randomized to receive ivermectin, 300 μg/kg of body weight per day for 5 days (n = 200) or placebo (n = 200). MAIN OUTCOMES AND MEASURES Primary outcome was time to resolution of symptoms within a 21-day follow-up period. Solicited adverse events and serious adverse events were also collected. RESULTS Among 400 patients who were randomized in the primary analysis population (median age, 37 years [interquartile range {IQR}, 29-48]; 231 women [58%]), 398 (99.5%) completed the trial. The median time to resolution of symptoms was 10 days (IQR, 9-13) in the ivermectin group compared with 12 days (IQR, 9-13) in the placebo group (hazard ratio for resolution of symptoms, 1.07 [95% CI, 0.87 to 1.32]; P = .53 by log-rank test). By day 21, 82% in the ivermectin group and 79% in the placebo group had resolved symptoms. The most common solicited adverse event was headache, reported by 104 patients (52%) given ivermectin and 111 (56%) who received placebo. The most common serious adverse event was multiorgan failure, occurring in 4 patients (2 in each group). CONCLUSION AND RELEVANCE Among adults with mild COVID-19, a 5-day course of ivermectin, compared with placebo, did not significantly improve the time to resolution of symptoms. The findings do not support the use of ivermectin for treatment of mild COVID-19, although larger trials may be needed to understand the effects of ivermectin on other clinically relevant outcomes.
Funding/Support: This study received an unrestricted grant from Centro de Estudios en Infectología Pediátrica (grant ScDi823).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Group Information
References
Ahmed, Hany, Youssef, Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic, doi:10.21203/rs.3.rs-100956/v1
Arévalo, Pagotto, Pórfido, Ivermectin reduces coronavirus infection in vivo: a mouse experimental model, doi:10.1101/2020.11.02.363242
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19: final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bray, Rayner, Noël, Jans, Wagstaff, Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors' responses, Antiviral Res, doi:10.1016/j.antiviral.2020.104805
Caly, Druce, Catton, Jans, Wagstaff, The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
De Melo, Lazarini, Larrous, Anti-COVID-19 efficacy of ivermectin in the golden hamster. bioRxiv, doi:10.1101/2020.11.21.392639
Hashim, Maulood, Rasheed, Fatak, Kabah et al., Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. medRxiv, doi:10.1101/2020.10.26.20219345
Karaca-Mandic, Georgiou, Sen, Cao, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19, JAMA Intern Med, doi:10.1056/NEJMoa2001282
Mahajan, Larkins-Pettigrew, Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties, J Public Health (Oxf), doi:10.1093/pubmed/fdaa070
Mastrangelo, Pezzullo, Burghgraeve, Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug, J Antimicrob Chemother, doi:10.1093/jac/dks147
Mitjà, Corbacho-Monné, Ubals, -CoV-2 RESEARCH GROUP. Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial, Clin Infect Dis
Momekov, Momekova, Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens, Biotechnol Biotechnol Equipment, doi:10.1080/13102818.2020.1775118
Niaee, Gheibi, Namdar, Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial, doi:10.21203/rs.3.rs-109670/v1
Omura, Ivermectin: 25 years and still going strong, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2007.08.023
Rajter, Sherman, Fatteh, Vogel, Sacks et al., Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study, Chest, doi:10.1016/j.chest.2020.10.009
Rodriguez, America's embrace of an unproven COVID treatment is hindering drug trials
Schmith, Zhou, Lohmer, Sanchez, Mejia-Fernandez et al., The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19, Clin Pharmacol Ther, doi:10.1002/cpt.1889
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant, doi:10.1016/j.healun.2020.03.012
Smit, Ochomo, Aljayyoussi, Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis, doi:10.1016/S1473-3099(18)30163-4
Spinner, Gottlieb, Criner, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2021.3071
Tay, Fraser, Chan, Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5: protection against all 4 DENV serotypes by the inhibitor Ivermectin, Antiviral Res, doi:10.1016/j.antiviral.2013.06.002
Wagstaff, Sivakumaran, Heaton, Harrich, Jans, Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus, Biochem J, doi:10.1042/BJ20120150
Yang, Atkinson, Wang, The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer, Antiviral Res, doi:10.1016/j.antiviral.2020.104760
DOI record:
{
"DOI": "10.1001/jama.2021.3071",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2021.3071",
"author": [
{
"affiliation": [
{
"name": "Centro de Estudios en Infectología Pediátrica, Cali, Colombia"
},
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
},
{
"name": "Clínica Imbanaco, Cali, Colombia"
}
],
"family": "López-Medina",
"given": "Eduardo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Centro de Estudios en Infectología Pediátrica, Cali, Colombia"
},
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
}
],
"family": "López",
"given": "Pío",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Universidad del Valle, Cali, Colombia"
},
{
"name": "State Health Department, Valle del Cauca, Colombia"
}
],
"family": "Hurtado",
"given": "Isabel C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Public Health, Universidad Icesi, Cali, Colombia"
}
],
"family": "Dávalos",
"given": "Diana M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
},
{
"name": "POHEMA (Pediatric Oncologist and Hematologist) Foundation, Cali, Colombia"
},
{
"name": "Cali’s Cancer Population-based Registry, Cali, Colombia"
}
],
"family": "Ramirez",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Universidad del Valle, Cali, Colombia"
},
{
"name": "Christus Sinergia Salud, Cali, Colombia"
}
],
"family": "Martínez",
"given": "Ernesto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Neurólogos de Occidente, Cali, Colombia"
}
],
"family": "Díazgranados",
"given": "Jesus A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
},
{
"name": "Department of Internal Medicine, Universidad del Valle, Cali, Colombia"
},
{
"name": "Clínica de Occidente, Cali, Colombia"
}
],
"family": "Oñate",
"given": "José M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Municipal Health Department, Cali, Colombia"
}
],
"family": "Chavarriaga",
"given": "Hector",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Caucaseco Scientific Research Center, Malaria Vaccine and Drug Development Center, Cali, Colombia"
}
],
"family": "Herrera",
"given": "Sócrates",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Universidad del Valle, Cali, Colombia"
}
],
"family": "Parra",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology, Universidad del Valle, Cali, Colombia"
}
],
"family": "Libreros",
"given": "Gerardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemato Oncólogos, Cali, Colombia"
}
],
"family": "Jaramillo",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hemato Oncólogos, Cali, Colombia"
}
],
"family": "Avendaño",
"given": "Ana C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Experts Committee, Valle del Cauca, Colombia"
}
],
"family": "Toro",
"given": "Dilian F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Municipal Health Department, Cali, Colombia"
}
],
"family": "Torres",
"given": "Miyerlandi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Health Department, Valle del Cauca, Colombia"
}
],
"family": "Lesmes",
"given": "Maria C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centro Médico Santuario, Cali, Colombia"
}
],
"family": "Rios",
"given": "Carlos A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Imbanaco, Cali, Colombia"
}
],
"family": "Caicedo",
"given": "Isabella",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
4
]
],
"date-time": "2021-03-04T17:30:28Z",
"timestamp": 1614879028000
},
"deposited": {
"date-parts": [
[
2021,
4,
14
]
],
"date-time": "2021-04-14T03:08:45Z",
"timestamp": 1618369725000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T17:20:37Z",
"timestamp": 1712596837821
},
"is-referenced-by-count": 260,
"issue": "14",
"issued": {
"date-parts": [
[
2021,
4,
13
]
]
},
"journal-issue": {
"issue": "14",
"published-print": {
"date-parts": [
[
2021,
4,
13
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2777389/jama_lpezmedina_2021_oi_210022_1618250230.41706.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1426",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
4,
13
]
]
},
"published-print": {
"date-parts": [
[
2021,
4,
13
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2007.08.023",
"article-title": "Ivermectin: 25 years and still going strong.",
"author": "Omura",
"doi-asserted-by": "publisher",
"first-page": "91",
"issue": "2",
"journal-title": "Int J Antimicrob Agents",
"key": "joi210022r1",
"volume": "31",
"year": "2008"
},
{
"DOI": "10.1016/j.antiviral.2020.104760",
"article-title": "The broad spectrum antiviral ivermectin targets the host nuclear transport importin a/ß1 heterodimer.",
"author": "Yang",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r2",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1042/BJ20120150",
"article-title": "Ivermectin is a specific inhibitor of importin a/ß-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus.",
"author": "Wagstaff",
"doi-asserted-by": "publisher",
"first-page": "851",
"issue": "3",
"journal-title": "Biochem J",
"key": "joi210022r3",
"volume": "443",
"year": "2012"
},
{
"DOI": "10.1093/jac/dks147",
"article-title": "Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug.",
"author": "Mastrangelo",
"doi-asserted-by": "publisher",
"first-page": "1884",
"issue": "8",
"journal-title": "J Antimicrob Chemother",
"key": "joi210022r4",
"volume": "67",
"year": "2012"
},
{
"DOI": "10.1016/j.antiviral.2013.06.002",
"article-title": "Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5: protection against all 4 DENV serotypes by the inhibitor Ivermectin.",
"author": "Tay",
"doi-asserted-by": "publisher",
"first-page": "301",
"issue": "3",
"journal-title": "Antiviral Res",
"key": "joi210022r5",
"volume": "99",
"year": "2013"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro.",
"author": "Caly",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r8",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1016/j.onehlt.2020.100148",
"article-title": "COVID-19 and the rush for self-medication and self-dosing with ivermectin: a word of caution.",
"author": "Molento",
"doi-asserted-by": "crossref",
"journal-title": "One Health",
"key": "joi210022r14",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal.",
"author": "Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"issue": "5",
"journal-title": "J Heart Lung Transplant",
"key": "joi210022r15",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1093/pubmed/fdaa070",
"article-title": "Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties.",
"author": "Mahajan",
"doi-asserted-by": "publisher",
"first-page": "445",
"issue": "3",
"journal-title": "J Public Health (Oxf)",
"key": "joi210022r16",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.3857",
"article-title": "Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states.",
"author": "Karaca-Mandic",
"doi-asserted-by": "publisher",
"first-page": "131",
"issue": "1",
"journal-title": "JAMA Intern Med",
"key": "joi210022r17",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi210022r18",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of COVID-19: final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi210022r19",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial.",
"author": "Spinner",
"doi-asserted-by": "publisher",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "joi210022r20",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72?314 cases from the Chinese Center for Disease Control and Prevention.",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA",
"key": "joi210022r23",
"volume": "323",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine for early treatment of adults with mild COVID-19: a randomized-controlled trial.",
"author": "Mitjà",
"journal-title": "Clin Infect Dis",
"key": "joi210022r24",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104805",
"article-title": "Ivermectin and COVID-19: a report in antiviral research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses.",
"author": "Bray",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "joi210022r25",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1080/13102818.2020.1775118",
"article-title": "Ivermectin as a potential COVID-19 treatment from the pharmacokinetic point of view: antiviral levels are not likely attainable with known dosing regimens.",
"author": "Momekov",
"doi-asserted-by": "publisher",
"first-page": "469",
"journal-title": "Biotechnol Biotechnol Equipment",
"key": "joi210022r26",
"volume": "34",
"year": "2020"
},
{
"article-title": "Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ICON Study.",
"author": "Rajter",
"journal-title": "Chest",
"key": "joi210022r27"
},
{
"DOI": "10.1002/cpt.v108.4",
"article-title": "The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19.",
"author": "Schmith",
"doi-asserted-by": "publisher",
"first-page": "762",
"issue": "4",
"journal-title": "Clin Pharmacol Ther",
"key": "joi210022r32",
"volume": "108",
"year": "2020"
},
{
"article-title": "Ivermectin as an adjunct in the treatment of refractory epilepsy [article in Spanish].",
"author": "Diazgranados-Sanchez",
"first-page": "303",
"issue": "7",
"journal-title": "Rev Neurol",
"key": "joi210022r33",
"volume": "65",
"year": "2017"
},
{
"DOI": "10.1016/S1473-3099(18)30163-4",
"article-title": "Safety and mosquitocidal efficacy of high-dose ivermectin when co-administered with dihydroartemisinin-piperaquine in Kenyan adults with uncomplicated malaria (IVERMAL): a randomised, double-blind, placebo-controlled trial.",
"author": "Smit",
"doi-asserted-by": "publisher",
"first-page": "615",
"issue": "6",
"journal-title": "Lancet Infect Dis",
"key": "joi210022r34",
"volume": "18",
"year": "2018"
},
{
"key": "joi210022r6",
"unstructured": "Frontline Covid-19 Critical Care Alliance. Accessed December 19, 2020. https://covid19criticalcare.com/"
},
{
"key": "joi210022r7",
"unstructured": "US Senate Committee on Homeland Security & Governmental Affairs. Early outpatient treatment: an essential part of a COVID-19 solution, part II. Accessed December 19, 2020. https://www.hsgac.senate.gov/early-outpatient-treatment-an-essential-part-of-a-covid-19-solution-part-ii"
},
{
"DOI": "10.1101/2020.11.21.392639",
"doi-asserted-by": "crossref",
"key": "joi210022r9",
"unstructured": "de Melo? GD, Lazarini? F, Larrous? F, . Anti-COVID-19 efficacy of ivermectin in the golden hamster.? bioRxiv. Preprint posted November 22, 2020. doi:10.1101/2020.11.21.392639"
},
{
"DOI": "10.1101/2020.11.02.363242",
"doi-asserted-by": "crossref",
"key": "joi210022r10",
"unstructured": "Arévalo? A, Pagotto? R, Pórfido? J, . Ivermectin reduces coronavirus infection in vivo: a mouse experimental model.? bioRxiv. Preprint posted November 2, 2020. doi:10.1101/2020.11.02.363242?"
},
{
"key": "joi210022r11",
"unstructured": "Ministerio de Salud, República del Perú. Resolución ministerial No. 270-2020-MINSA. Accessed December 19, 2020. https://cdn.www.gob.pe/uploads/document/file/694719/RM_270-2020-MINSA.PDF"
},
{
"key": "joi210022r12",
"unstructured": "Rodriguez Mega? E. Latin America’s embrace of an unproven COVID treatment is hindering drug trials. Nature. Accessed December 19, 2020. https://www.nature.com/articles/d41586-020-02958-2"
},
{
"key": "joi210022r13",
"unstructured": "Ministerio de Salud, Gobierno del Estado de Bolivia. Resolución ministerial No. 0259. Accessed December 19, 2020. https://www.minsalud.gob.bo/component/jdownloads/?task=download.send&id=425&catid=27&m=0&Itemid=646"
},
{
"key": "joi210022r21",
"unstructured": "World Health Organization. WHO R&D blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. Accessed December 20, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf"
},
{
"key": "joi210022r22",
"unstructured": "US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Published November 27, 2017. Accessed December 20, 2020. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf"
},
{
"DOI": "10.21203/rs.3.rs-100956/v1",
"doi-asserted-by": "crossref",
"key": "joi210022r28",
"unstructured": "Ahmed? E, Hany? B, Abo Youssef? S, ? Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic.? Research Square. Preprint posted November 17, 2020. doi:10.21203/rs.3.rs-100956/v1"
},
{
"DOI": "10.1101/2020.10.26.20219345",
"doi-asserted-by": "crossref",
"key": "joi210022r29",
"unstructured": "Hashim? HA, Maulood? MF, Rasheed? AM, Fatak? DF, Kabah? KK, Abdulamir? AS. Controlled randomized clinical trial on using ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq.? medRxiv. Preprint posted October 27, 2020. doi:10.1101/2020.10.26.20219345"
},
{
"DOI": "10.21203/rs.3.rs-109670/v1",
"doi-asserted-by": "crossref",
"key": "joi210022r30",
"unstructured": "Niaee? MS, Gheibi? N, Namdar? P, . Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial.? Research Square. Preprint posted November 24, 2020. doi:10.21203/rs.3.rs-109670/v1"
},
{
"key": "joi210022r31",
"unstructured": "Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection. Accessed December 21, 2020. https://clinicaltrials.gov/ct2/show/results/NCT04523831"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.739669574.793584506",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2777389"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of Ivermectin on Time to Resolution of Symptoms Among Adults With Mild COVID-19",
"type": "journal-article",
"volume": "325"
}
