1-year health outcomes associated with systemic corticosteroids for COVID-19: a longitudinal cohort study
et al., ERJ Open Research, doi:10.1183/23120541.00474-2024, ISRCTN10980107, Aug 2024
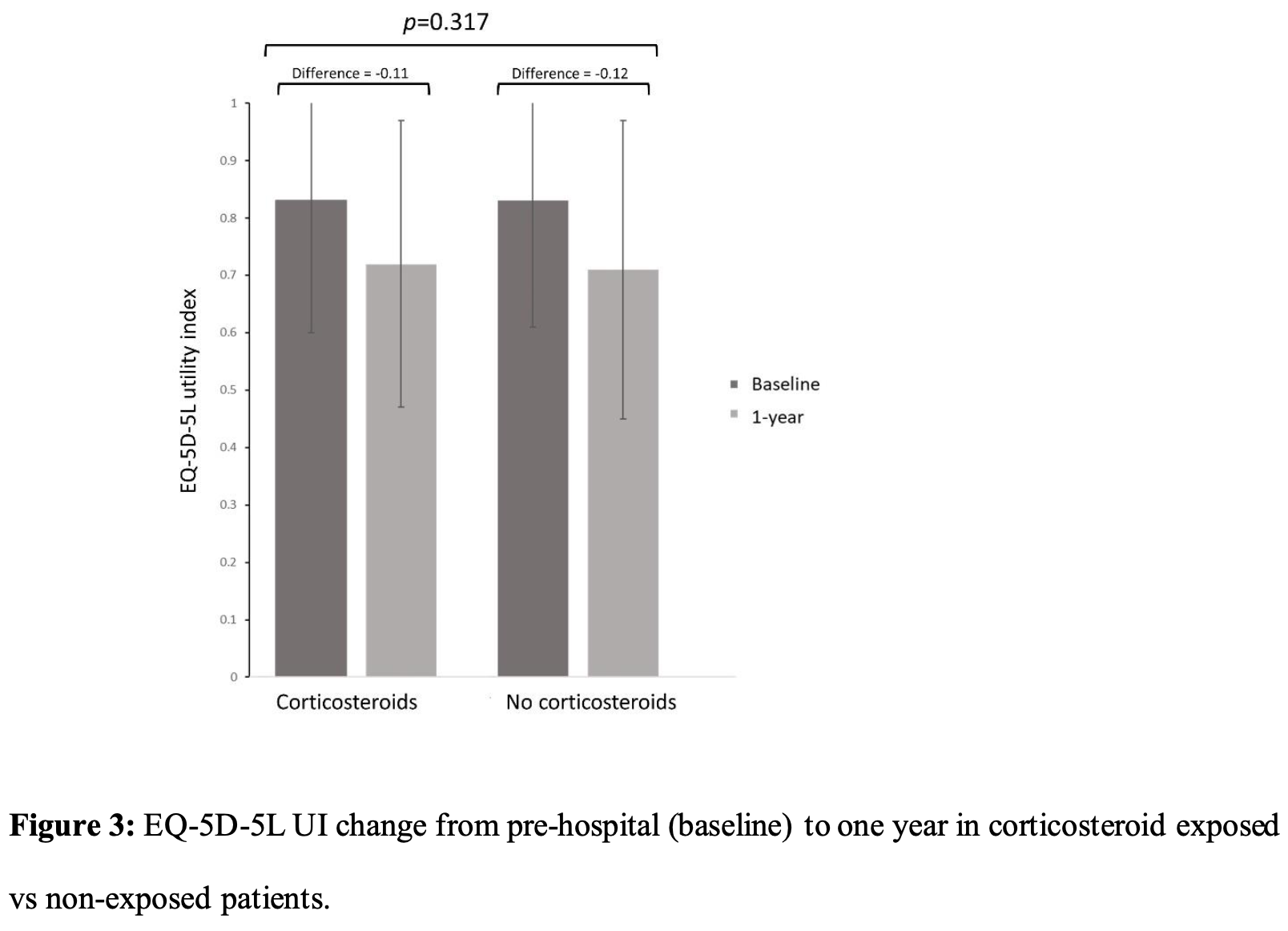
Retrospective 1,888 patients hospitalized with COVID-19 in the UK showing no significant difference in health-related quality of life or other patient reported outcomes, physical and mental health outcomes, and organ function one year after hospital discharge with systemic corticosteroid (dexamethasone) treatment during acute illness. Authors used propensity score weighting to balance corticosteroid exposed and non-exposed groups, and performed a sensitivity analysis using the larger ISARIC cohort to address potential selection and survivor biases.
Leavy et al., 22 Aug 2024, retrospective, United Kingdom, peer-reviewed, mean age 58.6, 73 authors, study period 1 February, 2020 - 31 March, 2021, trial ISRCTN10980107.
Contact: permissions@ersnet.org, re66@leicester.ac.uk.
One year health outcomes associated with systemic corticosteroids for COVID-19: a longitudinal cohort study
doi:10.1183/23120541.00474
Systemic corticosteroids given for acute COVID-19 do not affect health-related quality of life or other patient reported outcomes, physical and mental health outcomes, and organ function one year after hospital discharge
Conflict of interest statement: AART declares that their institute was awarded a fellowship from British Heart Foundation and grant funding from Heart Research UK and National Institute for Health and Care Research; payment for lectures and presentations received from Janssen-Cilag Ltd; support for attending meetings from Janssen-Cilag Ltd. AH declares that their institute was awarded funding from UK
Supplementary methodssensitivity analysis Sensitivity analyses were performed using the ISARIC (UK) dataset to address selection bias, treatment bias and survivor bias in PHOSP-COVID. First, a propensity score weighting for corticosteroid treatment was developed in the ISARIC (UK) cohort (survivors and non-survivors) using logistic regression with the following covariates: age, sex, obesity status, Index of Multiple Deprivation, WHO Clinical Progression Scale status, and the presence of specific comorbidities (cardiovascular disease, hypertension, chronic pulmonary disease, liver disease, renal disease, chronic neurological disease, malignancy, diabetes and rheumatological diseases). Second, using the PHOSP-COVID dataset, a prediction model was developed for EQ-5D-5L utility index at one year. Given the distribution of this measure, several modelling approaches were compared: linear regression, random forest, gradient boosted trees, and generalised linear models with gamma distribution (log link), poisson distribution, and zero-inflated poisson distribution. Based on..
References
Badenes, Bonet, Caguana, Vélez, Duran Jordà, Treatment of COVID-19 during the Acute Phase in Hospitalized Patients Decreases Post-Acute Sequelae of COVID-19, J Clin Med
Boglione, Meli, Poletti, Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?, Qjm
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial, Lancet Infect Dis
Catalán, Martí, Sota, Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission, J Med Virol
Davis, Mccorkell, Vogel, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
Elneima, Mcauley, Leavy, Cohort Profile: Post-hospitalisation COVID-19 study (PHOSP-COVID), medRxiv
Evans, Mcauley, Harrison, Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study, Lancet Respir Med
Finnigan, Cassar, Koziel, Efficacy and tolerability of an endogenous metabolic modulator (AXA1125) in fatigue-predominant long COVID: a single-centre, doubleblind, randomised controlled phase 2a pilot study, EClinicalMedicine
Granholm, Kjaer, Munch, Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia, Intensive Care Med
Group, Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study, Lancet Respir Med
Gsk, Sanofi, Regeneron, Roche, Genentech et al., GlaxoSmithKline, Veracyte, Resolution Therapeutics and Pliant; payment for lectures and presentations received from Boehringer Ingelheim, Chiesi, Roche, PatientMPower, AstraZeneca; payment for expert testimony from Pinsent Masons LLP; participation on a Data Safety Monitoring Board or Advisory Board for Boehringer Ingelheim, Galapagos, Vicore; leadership or fiduciary role for NuMedii and president for Action for Pulmonary Fibrosis. GC declares funding from GlaxoSmithKline and AstraZeneca; received honoraria for delivering talks from GSK, AZ, Chiesi, BI
Guralnik, Simonsick, Ferrucci, A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission, J Gerontol
Heal-Covid, HEAL-COVID study
Herdman, Gudex, Lloyd, Development and preliminary testing of the new fivelevel version of EQ-5D (EQ-5D-5L), Qual Life Res
Higgins, Berry, Lorenzi, Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial, Jama
Horby, Lim, Emberson, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Huang, Li, Gu, Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study, Lancet Respir Med
Johnson, Ulvenes, Øktedalen, Psychometric Properties of the General Anxiety Disorder 7-Item (GAD-7) Scale in a Heterogeneous Psychiatric Sample, Front Psychol
Levis, Benedetti, Thombs, Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis, Bmj
Madans, Loeb, Altman, Measuring disability and monitoring the UN Convention on the Rights of Persons with Disabilities: the work of the Washington Group on Disability Statistics, BMC Public Health
Mcnamara, Schneider, Love-Koh, Quality-Adjusted Life Expectancy Norms for the English Population, Value Health
Monk, Evans, Tear, Impact of Treatment of Hospitalised COVID-19 Patients With Inhaled Interferon Beta-1a (SNG001) on Long COVID Symptoms: Results From the SPRINTER trial, Open Forum Infectious Diseases
Nasreddine, Phillips, Bédirian, The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment, J Am Geriatr Soc
Nolan, Longworth, Lord, The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference, Thorax
Perego, Callard, Stras, Why the Patient-Made Term 'Long Covid' is needed, Wellcome Open Research
Phosp-Covid, The Post-hospitalisation COVID-19 study
Ramasawmy, Mu, Clutterbuck, STIMULATE-ICP-CAREINEQUAL (Symptoms, Trajectory, Inequalities and Management: Understanding Long-COVID to Address and Transform Existing Integrated Care Pathways) study protocol: Defining usual care and examining inequalities in Long Covid support, PLoS One
Singh, Morgan, Scott, Development of a shuttle walking test of disability in patients with chronic airways obstruction, Thorax
Toshner, Gamble, Baillie, Apixaban following discharge in hospitalised adults with COVID-19: Preliminary results from a multicentre, open-label, randomised controlled platform clinical trial, medRxiv
Watanabe, Iwagami, Yasuhara, Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis, Vaccine
Weathers, Litz, Keane, Palmieri, Marx et al., The PTSD Checklist for DSM-5 (PCL-5), Available from: Scale available from the National Center for PTSD at www
Xie, Choi, Al-Aly, Association of Treatment With Nirmatrelvir and the Risk of Post-COVID-19 Condition, JAMA Intern Med
Xie, Choi, Al-Aly, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, Bmj
Yellen, Cella, Webster, Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system, J Pain Symptom Manage
Yorke, Moosavi, Shuldham, Quantification of dyspnoea using descriptors: development and initial testing of the Dyspnoea-12, Thorax
DOI record:
{
"DOI": "10.1183/23120541.00474-2024",
"ISSN": [
"2312-0541"
],
"URL": "http://dx.doi.org/10.1183/23120541.00474-2024",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>In patients with coronavirus disease 2019 (COVID-19) requiring supplemental oxygen, dexamethasone reduces acute severity and improves survival, but longer-term effects are unknown. We hypothesised that systemic corticosteroid administration during acute COVID-19 would be associated with improved health-related quality of life (HRQoL) 1 year after discharge.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Adults admitted to hospital between February 2020 and March 2021 for COVID-19 and meeting current guideline recommendations for dexamethasone treatment were included using two prospective UK cohort studies (Post-hospitalisation COVID-19 and the International Severe Acute Respiratory and emerging Infection Consortium). HRQoL, assessed by the EuroQol-Five Dimensions–Five Levels utility index (EQ-5D-5L UI), pre-hospital and 1 year after discharge were compared between those receiving corticosteroids or not after propensity weighting for treatment. Secondary outcomes included patient-reported recovery, physical and mental health status, and measures of organ impairment. Sensitivity analyses were undertaken to account for survival and selection bias.</jats:p></jats:sec><jats:sec><jats:title>Findings</jats:title><jats:p>Of the 1888 participants included in the primary analysis, 1149 received corticosteroids. There was no between-group difference in EQ-5D-5L UI at 1 year (mean difference 0.004, 95% CI −0.026–0.034). A similar reduction in EQ-5D-5L UI was seen at 1 year between corticosteroid exposed and nonexposed groups (mean±<jats:sc>sd</jats:sc>change −0.12±0.22<jats:italic>versus</jats:italic>−0.11±0.22). Overall, there were no differences in secondary outcome measures. After sensitivity analyses modelled using a cohort of 109 318 patients admitted to hospital with COVID-19, EQ-5D-5L UI at 1 year remained similar between the two groups.</jats:p></jats:sec><jats:sec><jats:title>Interpretation</jats:title><jats:p>Systemic corticosteroids for acute COVID-19 have no impact on the large reduction in HRQoL 1 year after hospital discharge. Treatments to address the persistent reduction in HRQoL are urgently needed.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2024,
5,
15
]
]
},
"alternative-id": [
"10.1183/23120541.00474-2024"
],
"author": [
{
"affiliation": [],
"family": "Leavy",
"given": "Olivia C.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0195-0812",
"affiliation": [],
"authenticated-orcid": false,
"family": "Russell",
"given": "Richard J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harrison",
"given": "Ewen M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2707-2779",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lone",
"given": "Nazir I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kerr",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Docherty",
"given": "Annemarie B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sheikh",
"given": "Aziz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richardson",
"given": "Matthew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2480-8840",
"affiliation": [],
"authenticated-orcid": false,
"family": "Elneima",
"given": "Omer",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0453-7529",
"affiliation": [],
"authenticated-orcid": false,
"family": "Greening",
"given": "Neil J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Victoria Claire",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Houchen-Wolloff",
"given": "Linzy",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8997-0764",
"affiliation": [],
"authenticated-orcid": false,
"family": "McAuley",
"given": "Hamish J.C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saunders",
"given": "Ruth M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sereno",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shikotra",
"given": "Aarti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singapuri",
"given": "Amisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aul",
"given": "Raminder",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beirne",
"given": "Paul",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9578-2249",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bolton",
"given": "Charlotte E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Jeremy S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choudhury",
"given": "Gourab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diar Bakerly",
"given": "Nawar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Easom",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Echevarria",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fuld",
"given": "Jonathan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6863-585X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hart",
"given": "Nick",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7246-6040",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hurst",
"given": "John R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6308-6014",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jones",
"given": "Mark",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1508-8362",
"affiliation": [],
"authenticated-orcid": false,
"family": "Parekh",
"given": "Dhruv",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pfeffer",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Najib M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rowland-Jones",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shah",
"given": "Ajay M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wootton",
"given": "Dan G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3538-2451",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jolley",
"given": "Caroline",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0717-4551",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thompson",
"given": "A.A. Roger",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chalder",
"given": "Trudie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Melanie J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8566-0344",
"affiliation": [],
"authenticated-orcid": false,
"family": "De Soyza",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Geddes",
"given": "John R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenhalf",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heller",
"given": "Simon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2822-210X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Howard",
"given": "Luke",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8054-2293",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jacob",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jenkins",
"given": "R. Gisli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lord",
"given": "Janet M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3782-659X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Man",
"given": "Will D-C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McCann",
"given": "Gerry P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neubauer",
"given": "Stefan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7220-2555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Openshaw",
"given": "Peter J.M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7307-169X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Porter",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rowland",
"given": "Matthew J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scott",
"given": "Janet T.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9700-0418",
"affiliation": [],
"authenticated-orcid": false,
"family": "Semple",
"given": "Malcolm G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9834-0366",
"affiliation": [],
"authenticated-orcid": false,
"family": "Singh",
"given": "Sally J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "David",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3969-6143",
"affiliation": [],
"authenticated-orcid": false,
"family": "Toshner",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lewis",
"given": "Keir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heaney",
"given": "Liam G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Briggs",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Bang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thorpe",
"given": "Mathew",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0149-4869",
"affiliation": [],
"authenticated-orcid": false,
"family": "Quint",
"given": "Jennifer K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chalmers",
"given": "James D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ho",
"given": "Ling-Pei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1828-0058",
"affiliation": [],
"authenticated-orcid": false,
"family": "Horsley",
"given": "Alex",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7585-4743",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marks",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poinasamy",
"given": "Krisnah",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1239-9608",
"affiliation": [],
"authenticated-orcid": false,
"family": "Raman",
"given": "Betty",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4951-1867",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wain",
"given": "Louise V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brightling",
"given": "Christopher E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1667-868X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Evans",
"given": "Rachael A.",
"sequence": "additional"
}
],
"container-title": "ERJ Open Research",
"container-title-short": "ERJ Open Res",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"publications.ersnet.org"
]
},
"created": {
"date-parts": [
[
2024,
8,
22
]
],
"date-time": "2024-08-22T16:16:28Z",
"timestamp": 1724343388000
},
"deposited": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T19:43:43Z",
"timestamp": 1729194223000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "National Institute for Health Research"
},
{
"name": "MRC-UK Research and Innovation"
}
],
"indexed": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T20:10:18Z",
"timestamp": 1729195818866,
"version": "3.27.0"
},
"is-referenced-by-count": 0,
"issue": "5",
"issued": {
"date-parts": [
[
2024,
8,
22
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2024,
9,
30
]
]
},
"published-print": {
"date-parts": [
[
2024,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
22
]
],
"date-time": "2024-08-22T00:00:00Z",
"timestamp": 1724284800000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/23120541.00474-2024",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "00474-2024",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2024,
8,
22
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
22
]
]
},
"published-print": {
"date-parts": [
[
2024,
9
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.1"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.2"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.3"
},
{
"DOI": "10.1016/S0140-6736(22)01109-6",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.4"
},
{
"DOI": "10.1016/S2213-2600(21)00383-0",
"article-title": "Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study",
"author": "Evans",
"doi-asserted-by": "crossref",
"first-page": "1275",
"journal-title": "Lancet Respir Med",
"key": "2024101712303963000_10.5.00474-2024.5",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00127-8",
"article-title": "Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study",
"doi-asserted-by": "crossref",
"first-page": "761",
"journal-title": "Lancet Respir Med",
"key": "2024101712303963000_10.5.00474-2024.6",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00126-6",
"article-title": "Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "863",
"journal-title": "Lancet Respir Med",
"key": "2024101712303963000_10.5.00474-2024.7",
"volume": "10",
"year": "2022"
},
{
"key": "2024101712303963000_10.5.00474-2024.8",
"unstructured": "National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing the long-term effects of COVID-19. Date last accessed: 22 October 2023. Date last updated: 11 November 2021. www.nice.org.uk/guidance/ng188"
},
{
"key": "2024101712303963000_10.5.00474-2024.9",
"unstructured": "World Health Organization . Post COVID-19 condition (Long COVID). Date last accessed: October 22, 2023. Date last updated: 7 December 2021. www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition"
},
{
"DOI": "10.12688/wellcomeopenres.16307.1",
"article-title": "Why the patient-made term “long COVID” is needed",
"author": "Perego",
"doi-asserted-by": "crossref",
"first-page": "244",
"journal-title": "Wellcome Open Research",
"key": "2024101712303963000_10.5.00474-2024.10",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41579-022-00846-2",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.11"
},
{
"DOI": "10.1002/jmv.27296",
"article-title": "Corticosteroids for COVID-19 symptoms and quality of life at 1 year from admission",
"author": "Catalán",
"doi-asserted-by": "crossref",
"first-page": "205",
"journal-title": "J Med Virol",
"key": "2024101712303963000_10.5.00474-2024.12",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1001/jama.2022.23257",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.13"
},
{
"DOI": "10.1093/ije/dyad165",
"article-title": "Cohort profile: post-hospitalisation COVID-19 study (PHOSP-COVID)",
"author": "Elneima",
"doi-asserted-by": "crossref",
"first-page": "dyad165",
"journal-title": "Int J Epidemiol",
"key": "2024101712303963000_10.5.00474-2024.14",
"volume": "53",
"year": "2024"
},
{
"key": "2024101712303963000_10.5.00474-2024.15",
"unstructured": "International Severe Acute Respiratory and emerging Infection Consortium . COVID-19. Date last accessed: 22 August 2023. Date last updated: 2023. https://isaric.org/research/covid-19-clinical-research-resources/"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.16"
},
{
"key": "2024101712303963000_10.5.00474-2024.17",
"unstructured": "National Institute for Health and Care Excellence . COVID-19 rapid guideline: managing COVID-19. Date last accessed: 28 October 2023. Date last updated: 15 June 2022. www.nice.org.uk/guidance/ng191"
},
{
"DOI": "10.1007/s11136-011-9903-x",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.18"
},
{
"DOI": "10.1136/thx.2009.118521",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.19"
},
{
"DOI": "10.1016/S0885-3924(96)00274-6",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.20"
},
{
"DOI": "10.1186/1471-2458-11-S1-S4",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.21"
},
{
"DOI": "10.1136/thx.47.12.1019",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.22"
},
{
"DOI": "10.1093/geronj/49.2.M85",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.23"
},
{
"DOI": "10.1111/j.1532-5415.2005.53221.x",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.24"
},
{
"DOI": "10.3389/fpsyg.2019.01713",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.25"
},
{
"DOI": "10.1136/bmj.l1476",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.26"
},
{
"key": "2024101712303963000_10.5.00474-2024.27",
"unstructured": "Weathers FW , Litz BT , Keane TM , et al. The PTSD checklist for DSM-5 (PCL-5). Date last accessed: 5 August 2024. Date last updated: 6 December 2023. www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp"
},
{
"key": "2024101712303963000_10.5.00474-2024.28",
"unstructured": "Consumer Data Research Group . Index of Multiple Deprivation (IMD). Date last accessed 5 August 2024. Date last updated: 17 July 2024. https://data.cdrc.ac.uk/dataset/index-multiple-deprivation-imd"
},
{
"DOI": "10.1016/j.jval.2022.07.005",
"article-title": "Quality-adjusted life expectancy norms for the English population",
"author": "McNamara",
"doi-asserted-by": "crossref",
"first-page": "163",
"journal-title": "Value Health",
"key": "2024101712303963000_10.5.00474-2024.29",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1136/thoraxjnl-2015-207782",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.30"
},
{
"DOI": "10.1007/s00134-022-06677-2",
"article-title": "Long-term outcomes of dexamethasone 12 mg versus 6 mg in patients with COVID-19 and severe hypoxaemia",
"author": "Granholm",
"doi-asserted-by": "crossref",
"first-page": "580",
"journal-title": "Intensive Care Med",
"key": "2024101712303963000_10.5.00474-2024.31",
"volume": "48",
"year": "2022"
},
{
"DOI": "10.3390/jcm12124158",
"article-title": "Treatment of COVID-19 during the acute phase in hospitalized patients decreases post-acute sequelae of COVID-19",
"author": "Badenes Bonet",
"doi-asserted-by": "crossref",
"first-page": "4158",
"journal-title": "J Clin Med",
"key": "2024101712303963000_10.5.00474-2024.32",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.1093/qjmed/hcab297",
"article-title": "Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?",
"author": "Boglione",
"doi-asserted-by": "crossref",
"first-page": "865",
"journal-title": "QJM",
"key": "2024101712303963000_10.5.00474-2024.33",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2023.0743",
"article-title": "Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "554",
"journal-title": "JAMA Intern Med",
"key": "2024101712303963000_10.5.00474-2024.34",
"volume": "183",
"year": "2023"
},
{
"DOI": "10.1136/bmj-2022-074572",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.35"
},
{
"DOI": "10.1093/ofid/ofac492.1879",
"article-title": "LB1533. Impact of treatment of hospitalised COVID-19 patients with inhaled interferon beta-1a (SNG001) on long COVID symptoms: results from the SPRINTER trial",
"author": "Monk",
"doi-asserted-by": "crossref",
"first-page": "ofac492.1879",
"journal-title": "Open Forum Infect Dis",
"key": "2024101712303963000_10.5.00474-2024.36",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(23)00299-2",
"article-title": "Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial",
"author": "Bramante",
"doi-asserted-by": "crossref",
"first-page": "1119",
"journal-title": "Lancet Infect Dis",
"key": "2024101712303963000_10.5.00474-2024.37",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1101/2022.12.07.22283175",
"doi-asserted-by": "crossref",
"key": "2024101712303963000_10.5.00474-2024.38",
"unstructured": "Toshner MR , Gamble C , Baillie JK , et al. Apixaban following discharge in hospitalised adults with COVID-19: preliminary results from a multicentre, open-label, randomised controlled platform clinical trial. medRxiv 2022; preprint [https://doi.org/10.1101/2022.12.07.22283175]."
},
{
"key": "2024101712303963000_10.5.00474-2024.39",
"unstructured": "ClinicalTrials.gov . Helping alleviate the longer-term consequences of COVID-19 (HEAL-COVID). Date last updated: 23 July 2021. https://clinicaltrials.gov/study/NCT04801940/"
},
{
"DOI": "10.1016/j.eclinm.2023.101946",
"article-title": "Efficacy and tolerability of an endogenous metabolic modulator (AXA1125) in fatigue-predominant long COVID: a single-centre, double-blind, randomised controlled phase 2a pilot study",
"author": "Finnigan",
"doi-asserted-by": "crossref",
"first-page": "101946",
"journal-title": "EClinicalMedicine",
"key": "2024101712303963000_10.5.00474-2024.40",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0271978",
"doi-asserted-by": "publisher",
"key": "2024101712303963000_10.5.00474-2024.41"
},
{
"key": "2024101712303963000_10.5.00474-2024.42",
"unstructured": "PHOSP-COVID . The Post-hospitalisation COVID-19 study (PHOSP-COVID). Date last accessed: 3 October 2023. https://phosp.org/"
},
{
"DOI": "10.1016/j.vaccine.2023.02.008",
"article-title": "Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis",
"author": "Watanabe",
"doi-asserted-by": "crossref",
"first-page": "1783",
"journal-title": "Vaccine",
"key": "2024101712303963000_10.5.00474-2024.43",
"volume": "41",
"year": "2023"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "http://publications.ersnet.org/lookup/doi/10.1183/23120541.00474-2024"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "1-year health outcomes associated with systemic corticosteroids for COVID-19: a longitudinal cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1183/ers-crossmark-policy",
"volume": "10"
}