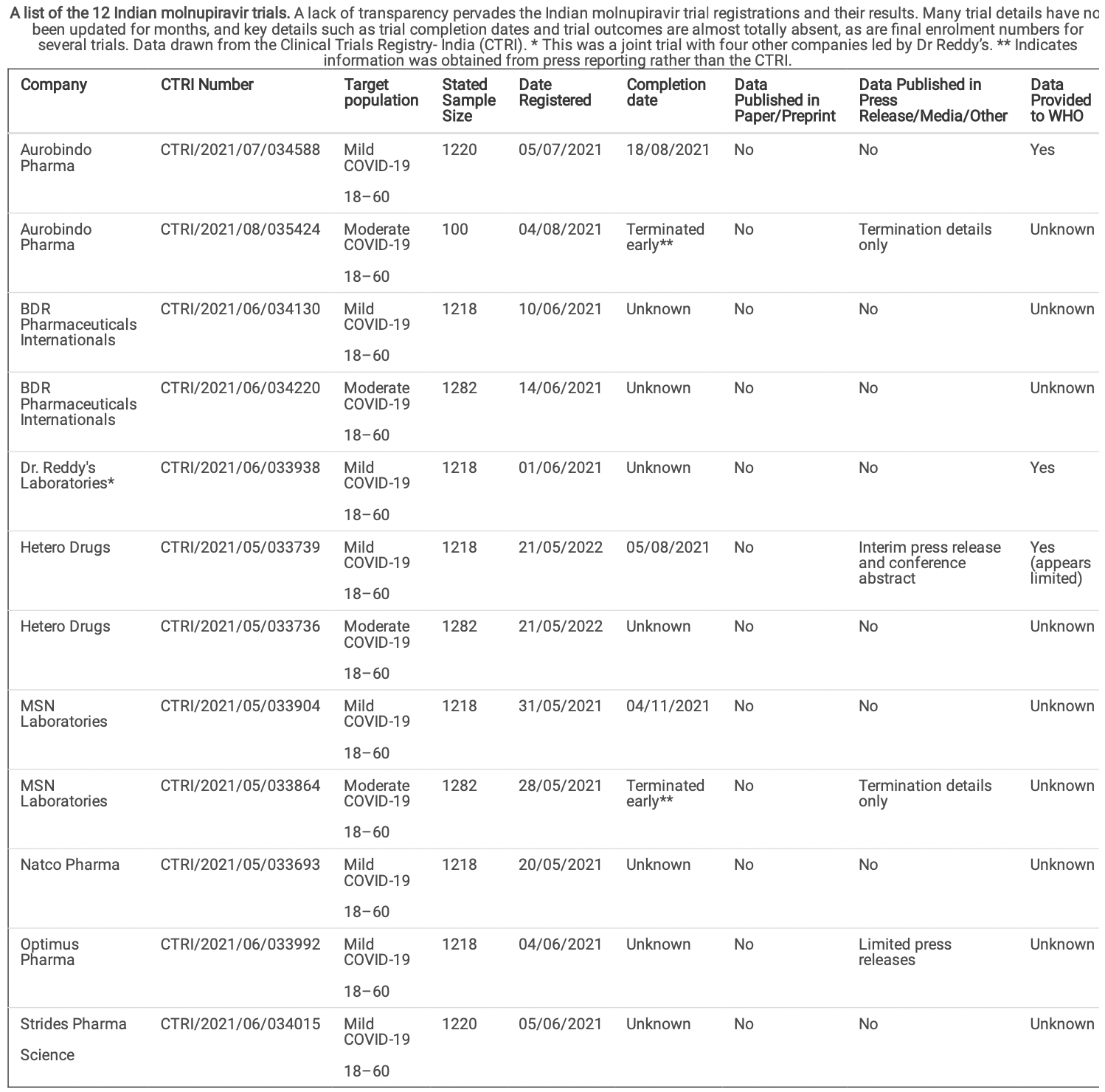
Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13,694 patients with meta-analysis
et al., Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkac440, Aug 2022 (preprint)
Meta analysis of molnupiravir trials showing 12 registered RCTs in India with only one presented at a conference, and two issuing press releases suggesting failure. Authors find that ~90% of the global data on molnupiravir has not been published. Authors also highlight issues with trials including the unexplained doubling in size and delayed presentation of PANORAMIC.
See also:1.
Currently there are 52 molnupiravir studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 9% lower [-2‑19%] |
| Ventilation | 11% lower [-19‑34%] |
| ICU admission | 3% lower [-18‑20%] |
| Hospitalization | 0% lower [-7‑7%] |
| Cases | 24% fewer [-1‑43%] |
Lawrence et al., 2 Aug 2022, peer-reviewed, 3 authors.
Contact: jackmlawrence@protonmail.com.
Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13,694 patients
doi:10.21203/rs.3.rs-1913200/v1
During the COVID-19 pandemic, Merck Sharp and Dohme (MSD) acquired the global licensing rights for molnupiravir. MSD allowed Indian manufacturers to produce the drug under voluntary license. Indian companies conducted local clinical trials to evaluate the e cacy and safety of molnupiravir.
Methods Searches of the Clinical Trials Registry-India (CTRI) were conducted to nd registered trials of molnupiravir in India. Subsequent investigations were performed to assess which clinical trials had been presented or published.
Results According to the CTRI, 12 randomised trials of molnupiravir were conducted in India, in 13,694 patients, starting in late May 2021. By July 2022, none of the 12 trials has been published, one was presented at a medical conference, and two were announced in press releases suggesting failure of treatment. Results from three trials were shared with the World Health Organisation. One of these three trials had many unexplained results, with effects of treatment signi cantly different from the MSD MOVE-OUT trial in a similar population.
Discussion The lack of results runs counter to established practices and leaves a situation where approximately 90% of the global data on molnupiravir has not been published in any form. Access to patient-level databases is required to investigate risks of bias or medical fraud.
is approaching 12 months since most of the 12 Indian trials concluded. While it is concerning that data from so many patients is missing, the circumstances are worsened because there is currently nothing to prevent the same situation from reoccurring with another drug.
Declarations The authors declare no con icts of interest. This research was funded by the International Treatment Preparedness Coalition / Make Medicines Affordable Campaign.
References
Babu, Visakhapatnam, Human Rights Forum demands action against Hetero drugs, The Times of India
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine
Brophy, Molnupiravir's authorisation was premature, BMJ
Buzz, Now, Covid pill from Optimus Pharma
Cdsco, COVID-19 related proposal under accelerated approval process
Devito, Bacon, Goldacre, Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study, Lancet
Drugs, Hetero Announces Interim Clinical Results from Phase III Clinical Trials of Molnupiravir conducted in India
Drugwatch, None
Drugwatch, Valsartan Lawsuit | Claims & What to Expect When Filing a Suit
Gov, Uk, None
Jefferson, Jones, Doshi, Mar, Hama et al., Neuraminidase inhibitors for preventing and treating in uenza in adults and children, Cochrane Database of Systematic Reviews, doi:10.1002/14651858.CD008965.pub4/full
Kaur, Icmr, Bhargava, Takes Strong Stand Against Molnupiravir for COVID -The Wire Science
Kumarasamy, Saha, Jindal, Singh, Podduturi et al., PHASE III TRIAL OF MOLNUPIRAVIR IN ADULTS WITH MILD SARS-CoV-2 INFECTION IN INDIA
Masyeni, Iqhrammullah, Frediansyah, Nainu, Tallei et al., Molnupiravir: A lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2-A narrative review, Journal of Medical Virology
Merck, Merck and Ridgeback Biotherapeutics Provide Update on Progress of Clinical Development Program for Molnupiravir, an Investigational Oral Therapeutic for the Treatment of Mild-to-Moderate COVID-19
Merck, Merck and Ridgeback's Investigational Oral Antiviral Molnupiravir Reduced the Risk of Hospitalization or Death by Approximately 50 Percent Compared to Placebo for Patients with Mild or Moderate COVID-19 in Positive Interim Analysis of Phase 3 Study
Merck, None
Merck, None
Merck, None
Msd, None
Panoramic, Recruitment begins for national community COVID-19 antiviral trial -PANORAMIC
Punj, Covid-19: Optimus concludes Phase 3 clinical trial of Molnupiravir oral capsule
Rajagopal, Expert panel defers approval for covid pill
Reddy, Laboratories, None
Research, De, None, Hetero Labs Limited Unit
Sheahan, Sims, Zhou, Graham, Hill et al., An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus, bioRxiv, doi:10.1101/2020.03.19.997890v1
Siemieniuk, Bartoszko, Zeraatkar, Kum, Qasim et al., Drug treatments for covid-19: living systematic review and network metaanalysis, BMJ
Siemieniuk, Bartoszko, Zeraatkar, Kum, Qasim et al., Drug treatments for covid-19: living systematic review and network metaanalysis, Supplementary Materials
Singh, Mitra, Arora, Two Indian drugmakers to end trials of generic Merck pill for moderate COVID-19, Reuters
Singh, Singh, Singh, Misra, Molnupiravir in COVID-19: A systematic review of literature, Diabetes Metab Syndr
Srivastava, Hetero Recalls Faulty Remdesivir Batch, Asks Hospitals & Stockists Not To Use -The Dialogue
Theprint, None
Theprint, Optimus announces Interim Clinical Results from Phase III Clinical Trials of Molnupiravir conducted in India
Thorlund, Sheldrick, Meyerowitz-Katz, Singh, Hill, Making Statistical Sense of the Molnupiravir MOVe-OUT Clinical Trial, The American Journal of Tropical Medicine and Hygiene
DOI record:
{
"DOI": "10.1093/jac/dkac440",
"ISSN": [
"0305-7453",
"1460-2091"
],
"URL": "http://dx.doi.org/10.1093/jac/dkac440",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>In response to the COVID-19 pandemic, Merck Sharp & Dohme (MSD) acquired the global licensing rights for the antiviral molnupiravir, promising affordable access via licensing deals. Numerous Indian pharmaceutical companies subsequently conducted trials of the drug. Registered trials of molnupiravir were searched on the Clinical Trials Registry–India (CTRI) and efforts made to detect resulting public data. Per the CTRI, 12 randomized trials of molnupiravir were conducted in 13 694 Indian patients, from mid-2021. By August 2022, only a preprint and medical conference presentation had resulted. Additionally, two trials were mentioned in press releases suggesting failure of treatment. The available data contain unexplained results that differ significantly from both the PANORAMIC and MSD MOVe-OUT trials. Approximately one-third of the global data on molnupiravir remain unpublished. We conducted a meta-analysis with four studies that provided results and observed that molnupiravir does not have a significant benefit for hospitalizations.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2027-5864",
"affiliation": [
{
"name": "St George’s, University of London , London , UK"
}
],
"authenticated-orcid": false,
"family": "Lawrence",
"given": "Jack M",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Imperial College London , London , UK"
}
],
"family": "Mirchandani",
"given": "Manya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology and Therapeutics, University of Liverpool , Liverpool , UK"
}
],
"family": "Hill",
"given": "Andrew",
"sequence": "additional"
}
],
"container-title": "Journal of Antimicrobial Chemotherapy",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
13
]
],
"date-time": "2022-12-13T23:37:34Z",
"timestamp": 1670974654000
},
"deposited": {
"date-parts": [
[
2023,
1,
8
]
],
"date-time": "2023-01-08T04:52:03Z",
"timestamp": 1673153523000
},
"funder": [
{
"name": "International Treatment Preparedness Coalition/Make Medicines Affordable Campaign"
}
],
"indexed": {
"date-parts": [
[
2023,
1,
9
]
],
"date-time": "2023-01-09T06:02:22Z",
"timestamp": 1673244142814
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
6
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
6
]
],
"date-time": "2023-01-06T00:00:00Z",
"timestamp": 1672963200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac440/48519081/dkac440.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jac/advance-article-pdf/doi/10.1093/jac/dkac440/48519081/dkac440.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
1,
6
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
6
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1126/scitranslmed.abb5883",
"article-title": "An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 and multiple endemic, epidemic and bat coronavirus",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "2023010804514408600_dkac440-B1",
"volume": "12",
"year": "2020"
},
{
"author": "Merck Sharp & Dohme",
"key": "2023010804514408600_dkac440-B2"
},
{
"author": "Merck",
"key": "2023010804514408600_dkac440-B3"
},
{
"author": "Merck",
"key": "2023010804514408600_dkac440-B4"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023010804514408600_dkac440-B5",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.4269/ajtmh.21-1339",
"article-title": "Making statistical sense of the molnupiravir MOVe-OUT clinical trial",
"author": "Thorlund",
"doi-asserted-by": "crossref",
"first-page": "1301",
"journal-title": "Am J Trop Med Hyg",
"key": "2023010804514408600_dkac440-B6",
"volume": "106",
"year": "2022"
},
{
"author": "Butler",
"key": "2023010804514408600_dkac440-B7"
},
{
"author": "US Food & Drug Administration",
"key": "2023010804514408600_dkac440-B8"
},
{
"author": "Merck Sharp & Dohme",
"key": "2023010804514408600_dkac440-B9"
},
{
"author": "Central Drugs Standard Control Organisation",
"key": "2023010804514408600_dkac440-B10"
},
{
"author": "Central Drugs Standard Control Organisation",
"key": "2023010804514408600_dkac440-B11"
},
{
"author": "Press Trust of India",
"key": "2023010804514408600_dkac440-B12"
},
{
"author": "Kaur",
"key": "2023010804514408600_dkac440-B13"
},
{
"author": "Press Trust India",
"key": "2023010804514408600_dkac440-B14"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B15"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B16"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B17"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B18"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B19"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B20"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B21"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B22"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B23"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B24"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B25"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B26"
},
{
"author": "Dr Reddy’s Laboratories",
"key": "2023010804514408600_dkac440-B27"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for COVID-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "crossref",
"first-page": "m2980",
"journal-title": "BMJ",
"key": "2023010804514408600_dkac440-B28",
"volume": "370",
"year": "2020"
},
{
"author": "Hetero Drugs",
"key": "2023010804514408600_dkac440-B29"
},
{
"author": "Kumarasamy",
"key": "2023010804514408600_dkac440-B30"
},
{
"author": "Tippabhotla",
"key": "2023010804514408600_dkac440-B31"
},
{
"author": "Clinical Trials Registry–India",
"key": "2023010804514408600_dkac440-B32"
},
{
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"journal-title": "BMJ",
"key": "2023010804514408600_dkac440-B33",
"volume": "370",
"year": "2022"
},
{
"author": "Singh",
"key": "2023010804514408600_dkac440-B34"
},
{
"author": "Natco Pharma Ltd",
"key": "2023010804514408600_dkac440-B35"
},
{
"author": "Natco Pharma Ltd",
"key": "2023010804514408600_dkac440-B36"
},
{
"author": "ThePrint",
"key": "2023010804514408600_dkac440-B37"
},
{
"DOI": "10.1016/j.dsx.2021.102329",
"article-title": "Molnupiravir in COVID-19: a systematic review of literature",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "102329",
"journal-title": "Diabetes Metab Syndr",
"key": "2023010804514408600_dkac440-B38",
"volume": "15",
"year": "2021"
},
{
"author": "Punj",
"key": "2023010804514408600_dkac440-B39"
},
{
"author": "Bizz Buzz",
"key": "2023010804514408600_dkac440-B40"
},
{
"author": "Rajagopal",
"key": "2023010804514408600_dkac440-B41"
},
{
"author": "CDSCO",
"key": "2023010804514408600_dkac440-B42"
},
{
"DOI": "10.1002/14651858.CD008965.pub4",
"article-title": "Neuraminidase inhibitors for preventing and treating influenza in adults and children",
"author": "Jefferson",
"doi-asserted-by": "crossref",
"journal-title": "Cochrane Database Syst Rev",
"key": "2023010804514408600_dkac440-B43",
"year": "2014"
},
{
"DOI": "10.1002/jmv.27730",
"article-title": "Molnupiravir: a lethal mutagenic drug against rapidly mutating severe acute respiratory syndrome coronavirus 2—a narrative review",
"author": "Masyeni",
"doi-asserted-by": "crossref",
"first-page": "3006",
"journal-title": "J Med Virol",
"key": "2023010804514408600_dkac440-B44",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1136/bmj.o443",
"article-title": "Molnupiravir’s authorisation was premature",
"author": "Brophy",
"doi-asserted-by": "crossref",
"first-page": "o443",
"journal-title": "BMJ",
"key": "2023010804514408600_dkac440-B45",
"volume": "376",
"year": "2022"
},
{
"article-title": "The mystery of India’s missing clinical trial results",
"author": "Dasgupta",
"journal-title": "BMJ",
"key": "2023010804514408600_dkac440-B46",
"volume": "371",
"year": "2020"
},
{
"author": "World Health Organization",
"key": "2023010804514408600_dkac440-B47"
},
{
"DOI": "10.1016/S0140-6736(19)33220-9",
"article-title": "Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study",
"author": "DeVito",
"doi-asserted-by": "crossref",
"first-page": "361",
"journal-title": "Lancet",
"key": "2023010804514408600_dkac440-B48",
"volume": "395",
"year": "2020"
},
{
"author": "Lawrence",
"key": "2023010804514408600_dkac440-B49"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jac/advance-article/doi/10.1093/jac/dkac440/6972450"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Pharmacology (medical)",
"Pharmacology",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Evaluation of publication bias for 12 clinical trials of molnupiravir to treat SARS-CoV-2 infection in 13 694 patients with meta-analysis",
"type": "journal-article"
}