A pharmacokinetic study and critical reappraisal of curcumin formulations enhancing bioavailability
et al., iScience, doi:10.1016/j.isci.2025.112575, May 2025
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
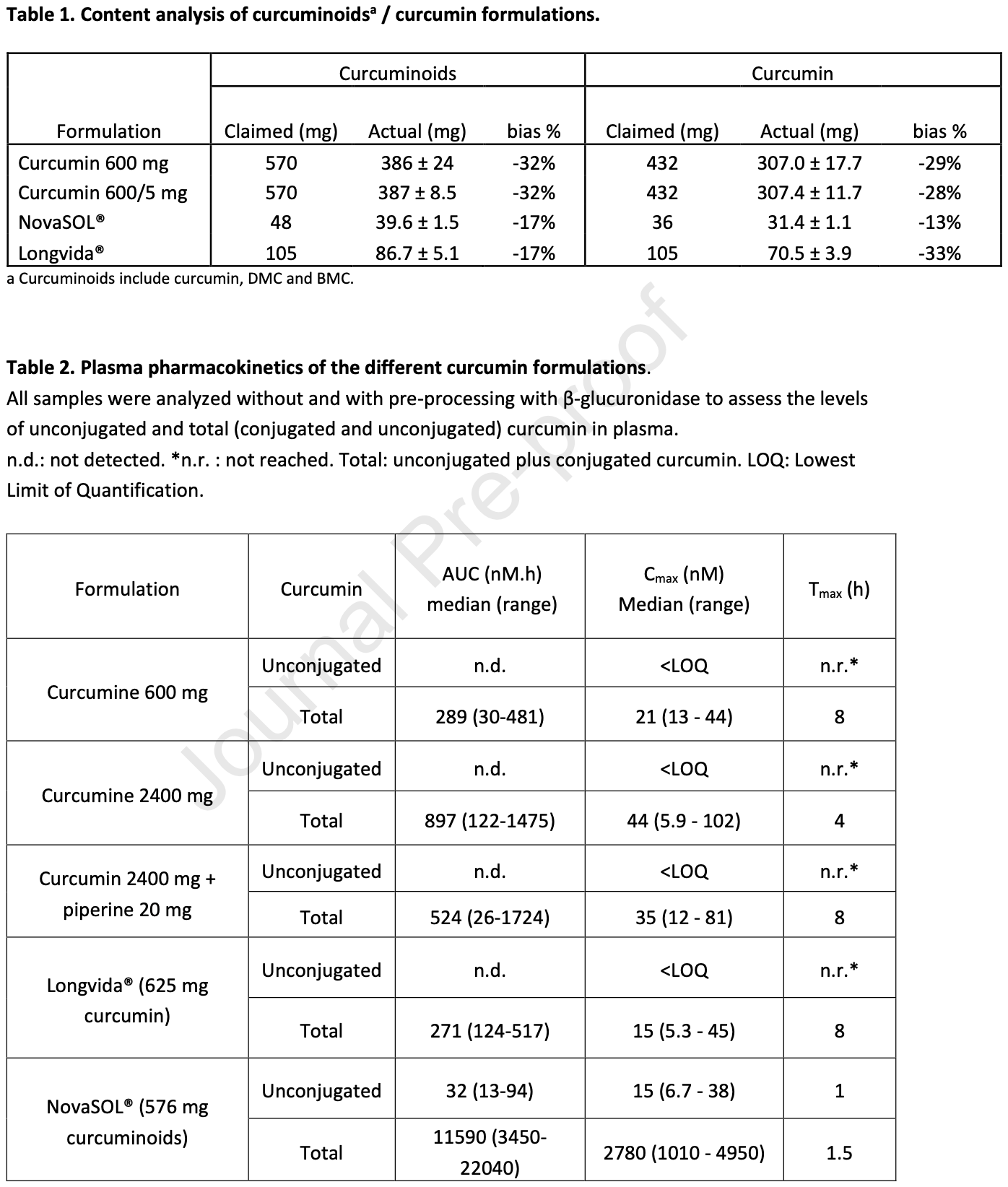
Crossover study of 9 healthy males comparing curcumin bioavailability from three commercial formulations (AOV, Longvida, NovaSOL). None of the three products tested contained the claimed amount of curcumin. Results showed plasma levels of unconjugated curcumin remained below 2nM with most formulations, and the best performer (NovaSOL) did not reach concentrations needed for biological effects in vitro. Conjugated curcumin (inactive form) exceeded 10nM with all formulations, but these metabolites have poor membrane permeability and limited bioactivity. Fecal recovery was high for all formulations except NovaSOL, suggesting better intestinal absorption with the latter, but still insufficient to achieve therapeutic plasma levels. The addition of piperine provided no significant benefit. Authors conclude that bioavailability claims should focus on unconjugated curcumin rather than conjugated forms, and that the specific formulations tested are unlikely to achieve therapeutic systemic levels.
Kroon et al., 3 May 2025, peer-reviewed, 5 authors.
Contact: m.kemper@apotheeka15.nl (corresponding author), o.v.tellingen@nki.nl.
A pharmacokinetic study and critical reappraisal of curcumin formulations enhancing bioavailability
iScience, doi:10.1016/j.isci.2025.112575
This independent crossover study assessed curcumin bioavailability and excretion in nine healthy males receiving three formulations (AOV, Longvida, NovaSOL) at about 570 mg, while AOV was also tested at 2280 mg, with and without piperine. Plasma levels of unconjugated curcumin remained below 2 nM in most cases, including high-dose AOV and piperine combinations. NovaSOL achieved the highest levels (6.7-38 nM at 30 min), but these rapidly declined and were still 100-fold lower than concentrations used in vitro to show biological effects. Curcumin conjugates exceeded 10 nM with all formulations, particularly NovaSOL, which showed 100-fold higher levels. Fecal recovery mainly included unconjugated curcuminoids and was high, except for NovaSOL, suggesting better intestinal absorption. However, even when using a formulation with enhanced uptake, plasma levels of unconjugated curcumin remained minimal. Piperine addition provided no benefit. The findings underscore that bioavailability claims should be based on unconjugated curcumin and not on its poorly membrane permeable conjugates.
Resource availability Lead Contact: Further information can be directed to the lead contact: Olaf van Tellingen (o.v.tellingen@nki.nl)
Materials Availability This study did not generate new unique reagents
Author Contributions Conceptualization, M.K., O.V.T., and E.M.K.; Methodology, M.K., O.V.T., and E.M.K.; Formal Analysis, M.K., O.V.T., and E.M.K.; Investigation, M.K.; Resources, M.K. and O.V.T.; Writing -Original Draft, M.K.; Writing -Review & Editing, M.K.,H.V.L., E.L.S, O.V.T., and E.M.K.; Visualization, M.K., O.V.T., and E.M.K.; Supervision, H.V.L., E.L.S, O.V.T., and E.M.K.; Project Administration, M.K., O.V.T., and E.M.K.; Funding Acquisition, E.M.K.
Declaration of interests The authors declare no competing interests.
METHOD DETAILS
Study procedures Participants were instructed to visit the AMC fasted (fasted for at least 4 hours before t=0) in the morning of day 1 (around 8:00 AM). They were allowed to consume water. Two hours after the curcumin intake, foods and beverages were provided by the investigators to prevent consumption of products containing curcumin/piperine. Blood samples were collected at t=0 (h), t=0.25 (h), t=0.5 (h), t=0.75 (h), t=1 (h), t=1.5 (h), t=2 (h), t=4 (h), t=8 (h), and t=24 (h) after intake. For the final blood and urine sample participants returned to the site at the t=0 (h) the next day. Urine was collected from t=0 (h) until 24 hours after intake, and feces were collected until 48 hours after intake. Participants were..
References
Aggarwal, Sung, Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets, Trends Pharmacol. Sci, doi:10.1016/j.tips.2008.11.002
Awolade, Kerru, Gummidi, Oluwakemi, Singh, Therapeutic significance of β-glucuronidase activity and its inhibitors: A review, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2019.111921
Banerjee, Pal, Penmetsa, Kathi, Girish et al., Novel Bioenhanced Curcumin With Mesalamine for Induction of Clinical and Endoscopic Remission in Mild-to-Moderate Ulcerative Colitis: a Randomized Double-Blind Placebo-controlled Pilot Study, J. Clin. Gastroenterol, doi:10.1097/MCG.0000000000001416
Bhardwaj, Glaeser, Becquemont, Klotz, Gupta et al., Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4, J. Pharmacol. Exp. Ther, doi:10.1124/jpet.102.034728
Brosková, Drábiková, Sotníková, Fialová, Knezl, Effect of plant polyphenols on ischemia-reperfusion injury of the isolated rat heart and vessels, Phytother. Res, doi:10.1002/ptr.4825
Coelho, Romi, Ferreira, Zaltman, Soares-Mota, The Use of Curcumin as a Complementary Therapy in Ulcerative Colitis: A Systematic Review of Randomized Controlled Clinical Trials, Nutrients, doi:10.3390/nu12082296
Cuomo, Appendino, Dern, Schneider, Mckinnon et al., Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation, J. Nat. Prod, doi:10.1021/np1007262
Dashnyam, Mudududdla, Hsieh, Lin, Lin et al., β-Glucuronidases of opportunistic bacteria are the major contributors to xenobioticinduced toxicity in the gut, Sci Rep, doi:10.1038/s41598-018-34678-z
Dytrych, Kejík, Hajduch, Kaplánek, Veselá et al., Therapeutic potential and limitations of curcumin as antimetastatic agent, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.114758
Epstein, Sanderson, Macdonald, Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies, Br. J. Nutr, doi:10.1017/s0007114509993667
Fança-Berthon, Tenon, Bouter-Banon, Manfré, Maudet et al., Pharmacokinetics of a Single Dose of Turmeric Curcuminoids Depends on Formulation: Results of a Human Crossover Study, J. Nutr, doi:10.1093/jn/nxab087
Flory, Sus, Haas, Jehle, Kienhöfer et al., Increasing post-digestive solubility of curcumin is the most successful strategy to improve its oral bioavailability: A randomized cross-over trial in healthy adults and In Vitro bioaccessibility experiments, Mol. Nutr. Food Res, doi:10.1002/mnfr.201300724
Gota, Maru, Soni, Gandhi, Kochar et al., Safety and Pharmacokinetics of a Solid Lipid Curcumin Particle Formulation in Osteosarcoma Patients and Healthy Volunteers, J. Agric. Food Chem, doi:10.1021/jf9024807
Heger, Van Golen, Broekgaarden, Michel, The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer, Pharmacol. Rev, doi:10.1124/pr.110.004044
Hoehle, Pfeiffer, Solyom, Metzler, Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver, J. Agric. Food Chem, doi:10.1021/jf058146a
Huang, Ma, Lu, Chang, Fisher et al., Effects of curcumin, demethoxycurcumin, bisdemethoxycurcumin and tetrahydrocurcumin on 12-O-tetradecanoylphorbol-13-acetate-induced tumor promotion, Carcinogenesis, doi:10.1093/carcin/16.10.2493
J O U R N A L P R E, None
Jamilian, Foroozanfard, Kavossian, Aghadavod, Shafabakhsh et al., Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial, Clin. Nutr. ESPEN
Jamwal, Bioavailable curcumin formulations: A review of pharmacokinetic studies in healthy volunteers, J. Integr. Med, doi:10.1016/j.joim.2018.07.001
Jayaprakasha, Mohan Rao, Sakariah, Chemistry and biological activities of C. longa, Trends Food Sci. Technol, doi:10.1016/j.tifs.2005.08.006
Jäger, Lowery, Calvanese, Joy, Purpura et al., Comparative absorption of curcumin formulations, Nutrition J, doi:10.1186/1475-2891-13-11
Kanai, Imaizumi, Otsuka, Sasaki, Hashiguchi et al., Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers, Cancer Chemother. Pharmacol, doi:10.1007/s00280-011-1673-1
Kedia, Bhatia, Thareja, Garg, Mouli et al., Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: Results from a randomized double blind placebo controlled trial, World J. Gastrointest. Pharmacol. Ther, doi:10.4292/wjgpt.v8.i2.147
Khosravi, Seifert, Clinical trials on curcumin in relation to its bioavailability and effect on malignant diseases: critical analysis, Naunyn Schmiedebergs Arch. Pharmacol, doi:10.1007/s00210-023-02825-7
Kocher, Schiborr, Behnam, Frank, The oral biovailability of curcuminoids in healthy humans is markedly enhanced by micellar solubilisation but not further improved by simultaneous ingestion of sesamin, ferulic acid, naringenin and xanthohumol, J. Funct. Foods, doi:10.1002/mnfr.201300724
Kroon, Berbee, Majait, Swart, Van Tellingen et al., Non-therapeutic plasma levels in individuals utilizing curcumin supplements in daily life, Front Nutr, doi:10.3389/fnut.2023.1267035
Kroon, Van Laarhoven, Swart, Kemper, Van Tellingen, A validated HPLC-MS/MS method for simultaneously analyzing curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetra-hydrocurcumin and piperine in human plasma, urine or feces, Heliyon, doi:10.1016/j.heliyon.2023.e15540
Kumar, Jacob, Subash, Maliakkal, Johannah et al., Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following the oral administration of a food-grade formulation with fenugreek dietary fibre: A randomised double-blind crossover study, J. Funct. Foods, doi:10.1016/j.jff.2016.01.039
Kunihiro, Luis, Brickey, Frye, Chow et al., Beta-Glucuronidase Catalyzes Deconjugation and Activation of Curcumin-Glucuronide in Bone, J. Nat. Prod, doi:10.1021/acs.jnatprod.8b00873
Lang, Salomon, Wu, Kopylov, Lahat et al., Curcumin add-on therapy for induction of remission in mildmoderate active Ulcerative Colitis: A multi-center, prospective, randomized, double-blind, placebo-controlled trial, J. Crohns Colitis
Liu, Liu, He, Liu, Cheng et al., A Comprehensive Review on the Benefits and Problems of Curcumin with Respect to Human Health, Molecules, doi:10.3390/molecules27144400
Mazzanti, Giacomo, Curcumin and Resveratrol in the Management of Cognitive Disorders: What is the Clinical Evidence?, Molecules, doi:10.3390/molecules21091243
Na, Li, Pan, Zhou, Sun et al., Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial, Molecular Nutr. Food Res, doi:10.1002/mnfr.201200131
Nelson, Dahlin, Bisson, Graham, Pauli et al., The Essential Medicinal Chemistry of Curcumin, J. Med. Chem, doi:10.1021/acs.jmedchem.6b00975
Ozawa, Imaizumi, Sumi, Hashimoto, Kanai et al., Curcumin beta-D-Glucuronide Plays an Important Role to Keep High Levels of Free-Form Curcumin in the Blood, Biol. Pharm. Bull, doi:10.1248/bpb.b17-00339
Pal, Sung, Prasad, Schuber, Prasad et al., Curcumin glucuronides: assessing the proliferative activity against human cell lines, Bioorg. Med. Chem, doi:10.1016/j.bmc.2013.11.006
Sadeghi, Mansoori, Shayesteh, Hashemi, The effect of curcumin supplementation on clinical outcomes and inflammatory markers in patients with ulcerative colitis, Phytother. Res, doi:10.1002/ptr.6581
Schiborr, Kocher, Behnam, Jandasek, Toelstede et al., The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes, Mol. Nutr. Food Res, doi:10.1002/mnfr.201300724
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Medica, doi:10.1055/s-2006-957450
Shoji, Nakagawa, Watanabe, Tsuduki, Yamada et al., Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells, Food Chem, doi:10.1016/j.foodchem.2013.11.102
Sudheeran, Jacob, Mulakal, Nair, Maliakel et al., Safety, Tolerance, and Enhanced Efficacy of a Bioavailable Formulation of Curcumin With Fenugreek Dietary Fiber on Occupational Stress: A Randomized, Double-Blind, Placebo-Controlled Pilot Study, J. Clin. Psychopharmacol, doi:10.1097/jcp.0000000000000508
Vareed, Kakarala, Ruffin, Crowell, Normolle et al., Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects, Cancer Epidemiol. Biomarkers Prev, doi:10.1158/1055-9965.Epi-07-2693
Zhou, Li, Hua, Dai, Yin et al., The role of tetrahydrocurcumin in disease prevention and treatment, Food & Funct, doi:10.1039/d3fo05739a
DOI record:
{
"DOI": "10.1016/j.isci.2025.112575",
"ISSN": [
"2589-0042"
],
"URL": "http://dx.doi.org/10.1016/j.isci.2025.112575",
"alternative-id": [
"S2589004225008363"
],
"article-number": "112575",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "A pharmacokinetic study and critical reappraisal of curcumin formulations enhancing bioavailability"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "iScience"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.isci.2025.112575"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Kroon",
"given": "Maurice A.G.M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "van Laarhoven",
"given": "Hanneke W.M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swart",
"given": "Eleonora L.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1037-6269",
"affiliation": [],
"authenticated-orcid": false,
"family": "van Tellingen",
"given": "Olaf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kemper",
"given": "E. Marleen",
"sequence": "additional"
}
],
"container-title": "iScience",
"container-title-short": "iScience",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T22:54:52Z",
"timestamp": 1746312892000
},
"deposited": {
"date-parts": [
[
2025,
5,
3
]
],
"date-time": "2025-05-03T22:54:53Z",
"timestamp": 1746312893000
},
"indexed": {
"date-parts": [
[
2025,
5,
4
]
],
"date-time": "2025-05-04T04:07:04Z",
"timestamp": 1746331624570,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
1
]
],
"date-time": "2025-05-01T00:00:00Z",
"timestamp": 1746057600000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
5,
1
]
],
"date-time": "2025-05-01T00:00:00Z",
"timestamp": 1746057600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
29
]
],
"date-time": "2025-04-29T00:00:00Z",
"timestamp": 1745884800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004225008363?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004225008363?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "112575",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
5
]
]
},
"published-print": {
"date-parts": [
[
2025,
5
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589004225008363"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A pharmacokinetic study and critical reappraisal of curcumin formulations enhancing bioavailability",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy"
}
