Efficacy of “Essential Iodine Drops” against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)
, Z., PLOS ONE, doi:10.1371/journal.pone.0254341, Jul 2021
PVP-I for COVID-19
15th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
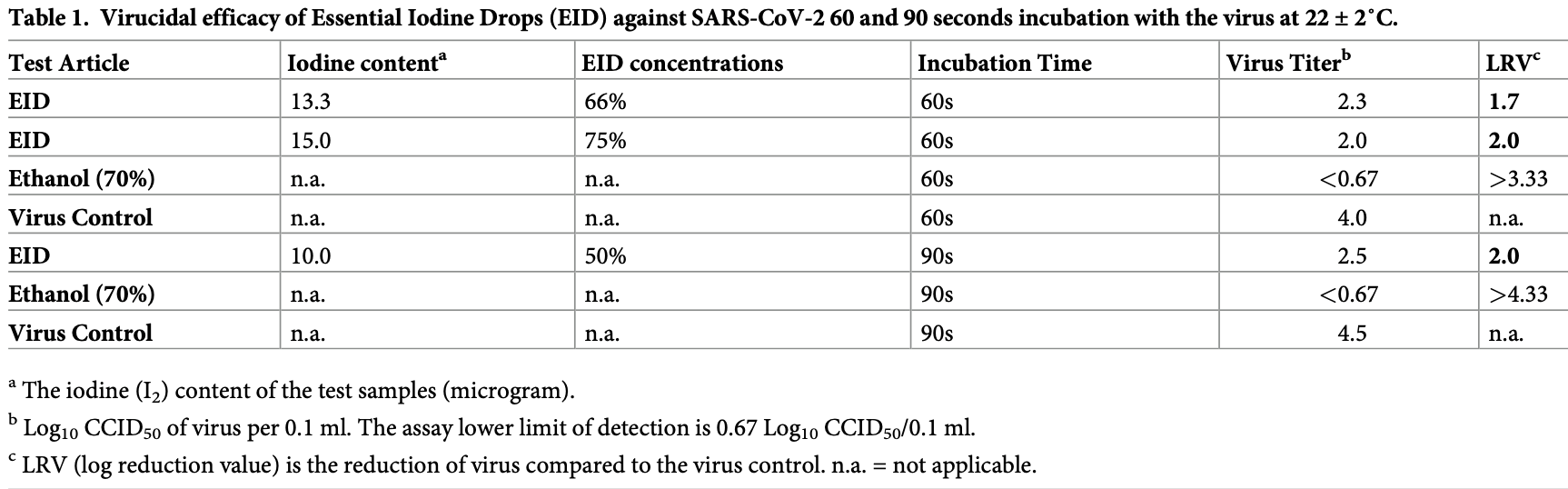
In vitro study showing 99% lower viral titer after 90 seconds with Essential Iodine Drops (EID), a formulation of Iodine-V. Author notes a potential safety advantage compared with PVP-I.
9 preclinical studies support the efficacy of povidone-iodine for COVID-19:
1.
Xu et al., Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro, Pathogens, doi:10.3390/pathogens10030272.
2.
Tucker et al., In vitro inactivation of SARS-CoV-2 with 0.5% povidone iodine nasal spray (Nasodine) at clinically relevant concentrations and timeframes using tissue culture and PCR based assays, bioRxiv, doi:10.1101/2021.01.31.426979.
3.
Pelletier et al., Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2), Ear, Nose & Throat Journal, doi:10.1177/0145561320957237.
4.
Frank et al., In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.3053.
5.
Meister et al., Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471.
6.
Anderson et al., Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease, Infectious Diseases and Therapy, doi:10.1007/s40121-020-00316-3.
7.
Hassandarvish et al., Povidone iodine gargle and mouthwash, British Dental Journal volume, doi:10.1038/s41415-020-1794-1.
Köntös et al., 9 Jul 2021, Hungary, peer-reviewed, 1 author.
Contact: zkontos@ioi-investment.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Efficacy of “Essential Iodine Drops” against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)
PLOS ONE, doi:10.1371/journal.pone.0254341
Background Aerosolization of respiratory droplets is considered the main route of coronavirus disease 2019 . Therefore, reducing the viral load of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) shed via respiratory droplets is potentially an ideal strategy to prevent the spread of the pandemic. The in vitro virucidal activity of intranasal Povidone-Iodine (PVP-I) has been demonstrated recently to reduce SARS-CoV-2 viral titres. This study evaluated the virucidal activity of the aqueous solution of Iodine-V (a clathrate complex formed by elemental iodine and fulvic acid) as in Essential Iodine Drops (EID) with 200 μg elemental iodine/ml content against SARS-CoV-2 to ascertain whether it is a better alternative to PVP-I.
Methods SARS-CoV-2 (USAWA1/2020 strain) virus stock was prepared by infecting Vero 76 cells (ATCC CRL-1587) until cytopathic effect (CPE). The virucidal activity of EID against SARS-CoV-2 was tested in three dilutions (1:1; 2:1 and 3:1) in triplicates by incubating at room temperature (22 ± 2˚C) for either 60 or 90 seconds. The surviving viruses from each sample were quantified by a standard end-point dilution assay.
Results EID (200 μg iodine/ml) after exposure for 60 and 90 seconds was compared to controls. In both cases, the viral titre was reduced by 99% (LRV 2.0). The 1:1 dilution of EID with virus reduced SARS-CoV-2 virus from 31,623 cell culture infectious dose 50% (CCID 50 ) to 316 CCID 50 within 90 seconds.
Conclusion Substantial reductions in LRV by Iodine-V in EID confirmed the activity of EID against SARS-CoV-2 in vitro, demonstrating that Iodine-V in EID is effective at inactivating the virus in vitro and therefore suggesting its potential application intranasally to reduce SARS-CoV-2 transmission from known or suspected COVID-19 patients.
Author Contributions Conceptualization: Zolta ´n Ko ¨nto ¨s.
References
Bidra, Pelletier, Westover, Frank, Brown et al., Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse, Journal of Prosthodontics, doi:10.1111/jopr.13209
Chu, Akl, Duda, Solo, Yaacoub et al., Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis, The Lancet
Dale, A Comparison of the Effectiveness of Tincture of Iodine and Potassium Iodide on Chronic Sinusitis Patients with Biofilm, Otolaryngology Open Access Journal
Dietz, Horve, Coil, Fretz, Eisen et al., Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission, mSystems, doi:10.1128/mSystems.00245-20
Frank, Capriotti, Brown, Tessema, Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era, Ear Nose Throat J, doi:10.1177/0145561320932318
Jia, Look, Shi, Hickey, Pewe et al., ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia, Journal of Virology, doi:10.1128/JVI.79.23.14614-14621.2005
Johnson, Morawska, Ristovski, Hargreaves, Mengersen et al., Modality of human expired aerosol size distributions, Journal of Aerosol Science
Kaiho, None, Iodine Chemistry and Applications
Kirk-Bayley, Challacombe, Sunkaraneni, Combes, The Use of Povidone Iodine Nasal Spray and Mouthwash During the Current COVID-19 Pandemic May Protect Healthcare Workers and Reduce Cross Infection, doi:10.2139/ssrn.3563092
Michavila-Gomez, Moreno-Palanques, Ferrer-Vazquez, Ferriols-Leisart, Bartolome, Anaphylactic reaction to povidone secondary to drug ingestion in a young child, Allergol Immunopathol (Madr)
Ong, Tan, Chia, Lee, Ng et al., Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient, Jama, doi:10.1001/jama.2020.3227
Pelletier, Tessema, Westover, Frank, Brown et al., Vitro Efficacy of Povidone-Iodine Nasal And Oral Antiseptic Preparations Against, doi:10.1101/2020.05.25.20110239
Reyazulla, Gopinath, Vaibhav, Raut, An unusual complication of late onset allergic contact dermatitis to povidone iodine in oral & maxillofacial surgery-a report of 2 cases, Eur Ann Allergy Clin Immunol
Santarpia, Rivera, Herrera, Morwitzer, Creager et al., Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center, doi:10.1101/2020.03.23.20039446
Somsen, Van Rijn, Kooij, Bem, Bonn, Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission, The Lancet Respiratory Medicine, doi:10.1016/S2213-2600%2820%2930245-9
Van Doremalen, Bushmaker, Morris, Holbrook, Gamble et al., Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1, The New England journal of medicine, doi:10.1056/NEJMc2004973
Wang, Pan, Tang, Ji, Shi, Mask use during COVID-19: A risk adjusted strategy, doi:10.1016/j.envpol.2020.115099
DOI record:
{
"DOI": "10.1371/journal.pone.0254341",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0254341",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Aerosolization of respiratory droplets is considered the main route of coronavirus disease 2019 (COVID-19). Therefore, reducing the viral load of Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) shed via respiratory droplets is potentially an ideal strategy to prevent the spread of the pandemic. The <jats:italic>in vitro</jats:italic> virucidal activity of intranasal Povidone-Iodine (PVP-I) has been demonstrated recently to reduce SARS-CoV-2 viral titres. This study evaluated the virucidal activity of the aqueous solution of Iodine-V (a clathrate complex formed by elemental iodine and fulvic acid) as in Essential Iodine Drops (EID) with 200 μg elemental iodine/ml content against SARS-CoV-2 to ascertain whether it is a better alternative to PVP-I.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods</jats:title>\n<jats:p>SARS-CoV-2 (USAWA1/2020 strain) virus stock was prepared by infecting Vero 76 cells (ATCC CRL-1587) until cytopathic effect (CPE). The virucidal activity of EID against SARS-CoV-2 was tested in three dilutions (1:1; 2:1 and 3:1) in triplicates by incubating at room temperature (22 ± 2°C) for either 60 or 90 seconds. The surviving viruses from each sample were quantified by a standard end-point dilution assay.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Results</jats:title>\n<jats:p>EID (200 μg iodine/ml) after exposure for 60 and 90 seconds was compared to controls. In both cases, the viral titre was reduced by 99% (LRV 2.0). The 1:1 dilution of EID with virus reduced SARS-CoV-2 virus from 31,623 cell culture infectious dose 50% (CCID<jats:sub>50</jats:sub>) to 316 CCID<jats:sub>50</jats:sub> within 90 seconds.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Conclusion</jats:title>\n<jats:p>Substantial reductions in LRV by Iodine-V in EID confirmed the activity of EID against SARS-CoV-2 in vitro, demonstrating that Iodine-V in EID is effective at inactivating the virus <jats:italic>in vitro</jats:italic> and therefore suggesting its potential application intranasally to reduce SARS-CoV-2 transmission from known or suspected COVID-19 patients.</jats:p>\n</jats:sec>",
"author": [
{
"affiliation": [],
"family": "Köntös",
"given": "Zoltán",
"sequence": "first"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2021,
7,
9
]
],
"date-time": "2021-07-09T18:09:39Z",
"timestamp": 1625854179000
},
"deposited": {
"date-parts": [
[
2021,
7,
9
]
],
"date-time": "2021-07-09T18:09:55Z",
"timestamp": 1625854195000
},
"editor": [
{
"affiliation": [],
"family": "Serra",
"given": "Raffaele",
"sequence": "first"
}
],
"indexed": {
"date-parts": [
[
2022,
8,
12
]
],
"date-time": "2022-08-12T04:49:53Z",
"timestamp": 1660279793762
},
"is-referenced-by-count": 2,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
7,
9
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2021,
7,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
9
]
],
"date-time": "2021-07-09T00:00:00Z",
"timestamp": 1625788800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0254341",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0254341",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
7,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
9
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1128/mSystems.00245-20",
"article-title": "2019 Novel Coronavirus (COVID-19) Pandemic: Built Environment Considerations To Reduce Transmission",
"author": "L Dietz",
"doi-asserted-by": "crossref",
"first-page": "e00245",
"journal-title": "mSystems",
"key": "pone.0254341.ref001",
"volume": "5",
"year": "2020"
},
{
"author": "J Santarpia",
"journal-title": "Transmission Potential of SARS-CoV-2 in Viral Shedding Observed at the University of Nebraska Medical Center",
"key": "pone.0254341.ref002",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2004973",
"article-title": "Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1",
"author": "N van Doremalen",
"doi-asserted-by": "crossref",
"first-page": "1564",
"journal-title": "The New England journal of medicine",
"key": "pone.0254341.ref003",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.3227",
"article-title": "Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient",
"author": "SWX Ong",
"doi-asserted-by": "crossref",
"first-page": "1610",
"journal-title": "Jama",
"key": "pone.0254341.ref004",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.jaerosci.2011.07.009",
"article-title": "Modality of human expired aerosol size distributions",
"author": "GR Johnson",
"doi-asserted-by": "crossref",
"first-page": "839",
"journal-title": "Journal of Aerosol Science",
"key": "pone.0254341.ref005",
"volume": "42",
"year": "2011"
},
{
"DOI": "10.1016/S2213-2600(20)30245-9",
"article-title": "Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission",
"author": "GA Somsen",
"doi-asserted-by": "crossref",
"first-page": "658",
"journal-title": "The Lancet Respiratory Medicine",
"key": "pone.0254341.ref006",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31142-9",
"article-title": "Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis",
"author": "DK Chu",
"doi-asserted-by": "crossref",
"first-page": "1973",
"journal-title": "The Lancet",
"key": "pone.0254341.ref007",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.envpol.2020.115099",
"article-title": "Mask use during COVID-19: A risk adjusted strategy",
"author": "J Wang",
"doi-asserted-by": "crossref",
"first-page": "115099",
"journal-title": "Environmental pollution (Barking, Essex: 1987)",
"key": "pone.0254341.ref008",
"volume": "266",
"year": "2020"
},
{
"article-title": "The Use of Povidone Iodine Nasal Spray and Mouthwash During the Current COVID-19 Pandemic May Protect Healthcare Workers and Reduce Cross Infection",
"author": "J Kirk-Bayley",
"journal-title": "SSRN Electronic Journal",
"key": "pone.0254341.ref009",
"year": "2020"
},
{
"article-title": "A Comparison of the Effectiveness of Tincture of Iodine and Potassium Iodide on Chronic Sinusitis Patients with Biofilm",
"author": "H Dale",
"journal-title": "Otolaryngology Open Access Journal",
"key": "pone.0254341.ref010",
"volume": "2",
"year": "2017"
},
{
"article-title": "Povidone-Iodine Use in Sinonasal and Oral Cavities: A Review of Safety in the COVID-19 Era",
"author": "S Frank",
"journal-title": "Ear Nose Throat J",
"key": "pone.0254341.ref011",
"year": "2020"
},
{
"article-title": "In Vitro Efficacy of Povidone-Iodine Nasal And Oral Antiseptic Preparations Against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)",
"author": "J Pelletier",
"journal-title": "medRxiv",
"key": "pone.0254341.ref012",
"year": "2020"
},
{
"DOI": "10.1111/jopr.13209",
"article-title": "Rapid In-Vitro Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Using Povidone-Iodine Oral Antiseptic Rinse",
"author": "AS Bidra",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Journal of Prosthodontics",
"key": "pone.0254341.ref013",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1128/JVI.79.23.14614-14621.2005",
"article-title": "ACE2 receptor expression and severe acute respiratory syndrome coronavirus infection depend on differentiation of human airway epithelia",
"author": "HP Jia",
"doi-asserted-by": "crossref",
"first-page": "14614",
"journal-title": "Journal of Virology",
"key": "pone.0254341.ref014",
"volume": "79",
"year": "2005"
},
{
"author": "Kaiho Tatsuo",
"first-page": "390",
"key": "pone.0254341.ref015",
"volume-title": "Iodine Chemistry and Applications",
"year": "2015"
},
{
"article-title": "An unusual complication of late onset allergic contact dermatitis to povidone iodine in oral & maxillofacial surgery—a report of 2 cases",
"author": "MA Reyazulla",
"first-page": "157",
"journal-title": "Eur Ann Allergy Clin Immunol",
"key": "pone.0254341.ref016",
"volume": "46",
"year": "2014"
},
{
"DOI": "10.1016/j.aller.2011.06.005",
"article-title": "Anaphylactic reaction to povidone secondary to drug ingestion in a young child",
"author": "AV Michavila-Gomez",
"doi-asserted-by": "crossref",
"first-page": "259",
"journal-title": "Allergol Immunopathol (Madr)",
"key": "pone.0254341.ref017",
"volume": "40",
"year": "2012"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0254341"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Efficacy of “Essential Iodine Drops” against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "16"
}
