Is PHASTR faster? A target trial emulation case study in the N3C
et al., Journal of Clinical and Translational Science, doi:10.1017/cts.2024.1162, Apr 2025
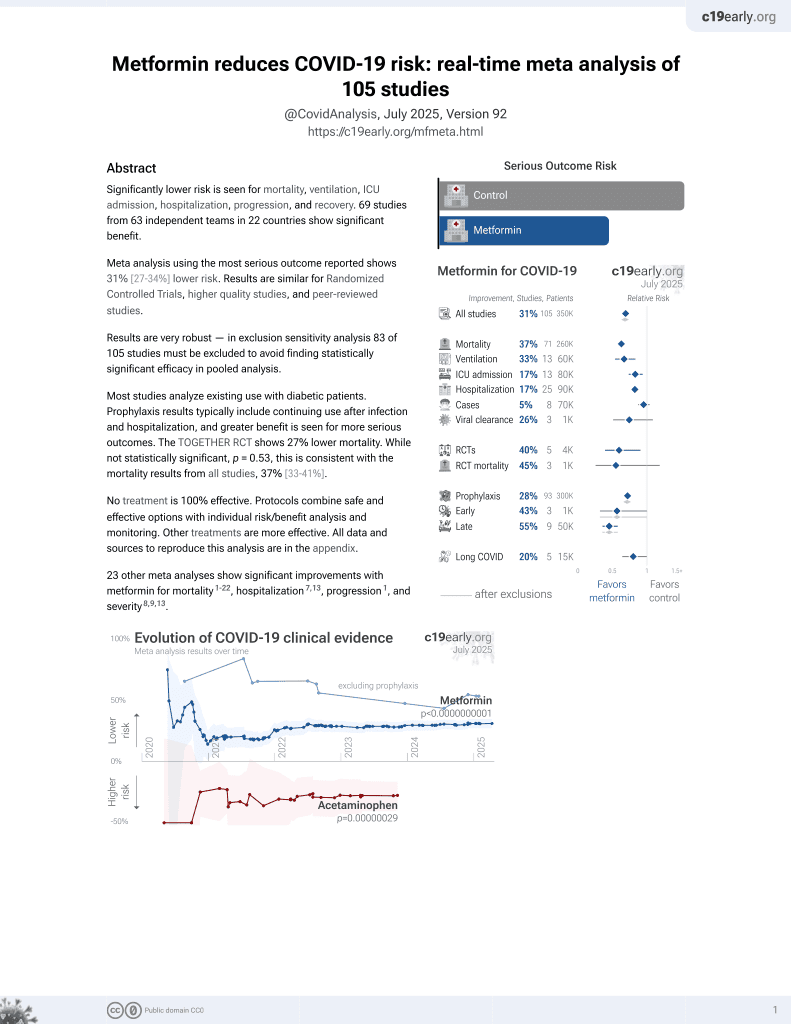
Metformin for COVID-19
3rd treatment shown to reduce risk in
July 2020, now with p < 0.00000000001 from 110 studies.
Lower risk for mortality, ventilation, ICU, hospitalization, progression, recovery, and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
N3C target trial emulation study with 9,660 adult COVID-19 patients, showing metformin associated with lower risk of long COVID or death.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
long COVID or death, 53.0% lower, RR 0.47, p = 0.02, treatment 248, control 248, adjusted per study, day 180.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Johnson et al., 11 Apr 2025, retrospective, USA, peer-reviewed, 7 authors.
595 Is PHASTR faster? A target trial emulation case study in the N3C
Journal of Clinical and Translational Science, doi:10.1017/cts.2024.1162
OBJECTIVES/GOALS: Team science (TS) competency is important for translational science team collaboration. However, there are few educators available to assist teams. Asynchronous learning is an effective strategy for delivering TS content. The goal of this project is to expand TS education by providing online access to our learners using online modules. METHODS/STUDY POPULATION: The Collaboration and Team Science (CaTS) team at the University of Cincinnati provides a robust TS education and training program. As the need for team science gains recognition, CaTS has received increased requests for services, leading to a need to broaden TS offerings. To address this demand, the CaTS team created "Team Science 101," an online, asynchronous, series of 15 modules covering basic team science concepts. Each module consists of an educational recording lasting an average of 20 minutes, optional topic resources, pre-and post-module surveys assessing learners' confidence and satisfaction, post-module knowledge checks, and evaluation questions. Upon completing all modules, participants receive a completion certificate. RESULTS/ANTICIPATED RESULTS: TS 101 will be piloted with a group of participants who expressed interest in asynchronous TS content and will be adjusted based on the feedback received. The associated pre-and post-module survey, post-module knowledge check, and evaluation questions will be monitored to determine learning levels and improve TS 101 overall. Canvas is the educational platform that houses these modules, allowing for participant followup and scalable dissemination. The CaTS team plans to disseminate TS 101 nationally and internationally for anyone interested in this resource. DISCUSSION/SIGNIFICANCE OF IMPACT: There is a national effort to collect and curate TS education, training, and toolkits. TS 101 will be a useful educational tool that will expand the reach of team science educators, provide the foundation for educators to explore topics more deeply by building on the module topics, and provide education to broader audiences who lack access to TS experts.
DOI record:
{
"DOI": "10.1017/cts.2024.1162",
"ISSN": [
"2059-8661"
],
"URL": "http://dx.doi.org/10.1017/cts.2024.1162",
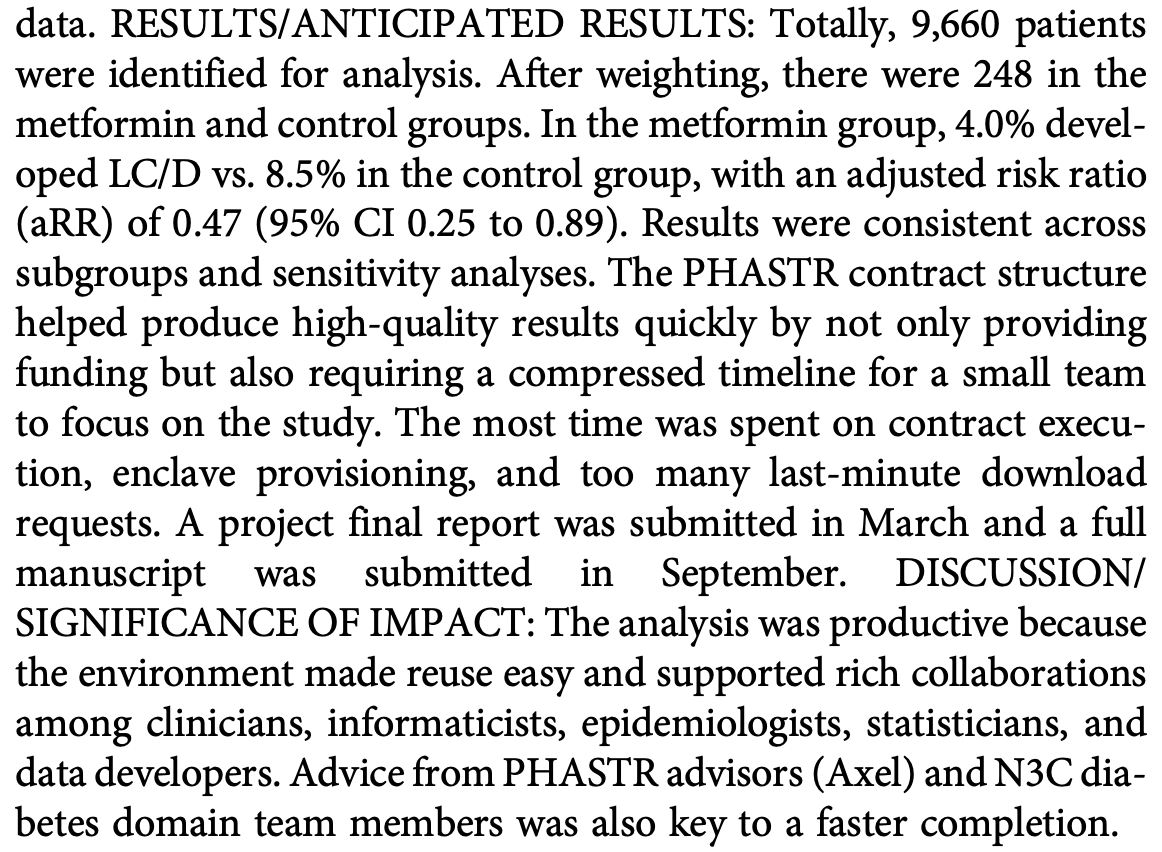
"abstract": "<jats:p><jats:bold>Objectives/Goals:</jats:bold> Our study team won a Public Health Answers to Speed Tractable Results (PHASTR) contract to conduct a target trial emulation to answer “Does metformin show a reduction of severe outcomes of COVID-19 or of Long COVID in the N3C Data Enclave?” We quickly delivered an answer due to productive technical and collaboration support in the N3C. <jats:bold>Methods/Study Population:</jats:bold> Our analytic plan was updated based on helpful feedback from the PHASTR program. We performed a trial emulation analysis using the N3C data, comparing adult new users of metformin to controls prescribed fluvoxamine, fluticasone, ivermectin, or montelukast. The composite outcome was Long COVID or Death (LC/D) within 180 days of COVID infection. We used entropy balancing to estimate the average treatment effect with a weighted log-linear model. Productivity was enhanced by reusing code workbooks and validated codesets from related N3C projects. The team of 4 (physician, informaticist, data programmer, and statistician) and key unpaid advisors spent 10 weeks developing and analyzing the data. <jats:bold>Results/Anticipated Results:</jats:bold> Totally, 9,660 patients were identified for analysis. After weighting, there were 248 in the metformin and control groups. In the metformin group, 4.0% developed LC/D vs. 8.5% in the control group, with an adjusted risk ratio (aRR) of 0.47 (95% CI 0.25 to 0.89). Results were consistent across subgroups and sensitivity analyses. The PHASTR contract structure helped produce high-quality results quickly by not only providing funding but also requiring a compressed timeline for a small team to focus on the study. The most time was spent on contract execution, enclave provisioning, and too many last-minute download requests. A project final report was submitted in March and a full manuscript was submitted in September. <jats:bold>Discussion/Significance of Impact:</jats:bold> The analysis was productive because the environment made reuse easy and supported rich collaborations among clinicians, informaticists, epidemiologists, statisticians, and data developers. Advice from PHASTR advisors (Axel) and N3C diabetes domain team members was also key to a faster completion.</jats:p>",
"alternative-id": [
"S2059866124011622"
],
"author": [
{
"affiliation": [],
"family": "Johnson",
"given": "Steve",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bramante",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stürmer",
"given": "Til",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buse",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiggen",
"given": "Talia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huling",
"given": "Jared",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Toler",
"given": "Andrew",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical and Translational Science",
"container-title-short": "J. Clin. Trans. Sci.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
26
]
],
"date-time": "2025-03-26T03:58:20Z",
"timestamp": 1742961500000
},
"deposited": {
"date-parts": [
[
2025,
4,
27
]
],
"date-time": "2025-04-27T09:57:02Z",
"timestamp": 1745747822000
},
"indexed": {
"date-parts": [
[
2025,
4,
27
]
],
"date-time": "2025-04-27T10:10:04Z",
"timestamp": 1745748604771,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issue": "s1",
"issued": {
"date-parts": [
[
2025,
4
]
]
},
"journal-issue": {
"issue": "s1",
"published-print": {
"date-parts": [
[
2025,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "unspecified",
"delay-in-days": 10,
"start": {
"date-parts": [
[
2025,
4,
11
]
],
"date-time": "2025-04-11T00:00:00Z",
"timestamp": 1744329600000
}
}
],
"link": [
{
"URL": "https://www.cambridge.org/core/services/aop-cambridge-core/content/view/S2059866124011622",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "56",
"original-title": [],
"page": "175-176",
"prefix": "10.1017",
"published": {
"date-parts": [
[
2025,
4
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
11
]
]
},
"published-print": {
"date-parts": [
[
2025,
4
]
]
},
"publisher": "Cambridge University Press (CUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cambridge.org/core/product/identifier/S2059866124011622/type/journal_article"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "595 Is PHASTR faster? A target trial emulation case study in the N3C",
"type": "journal-article",
"volume": "9"
}