Prevention of COVID-19 Following a Single Intramuscular Administration of Adintrevimab: Results From a Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial (EVADE)
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad314, EVADE PrEP, NCT04859517, Jun 2023
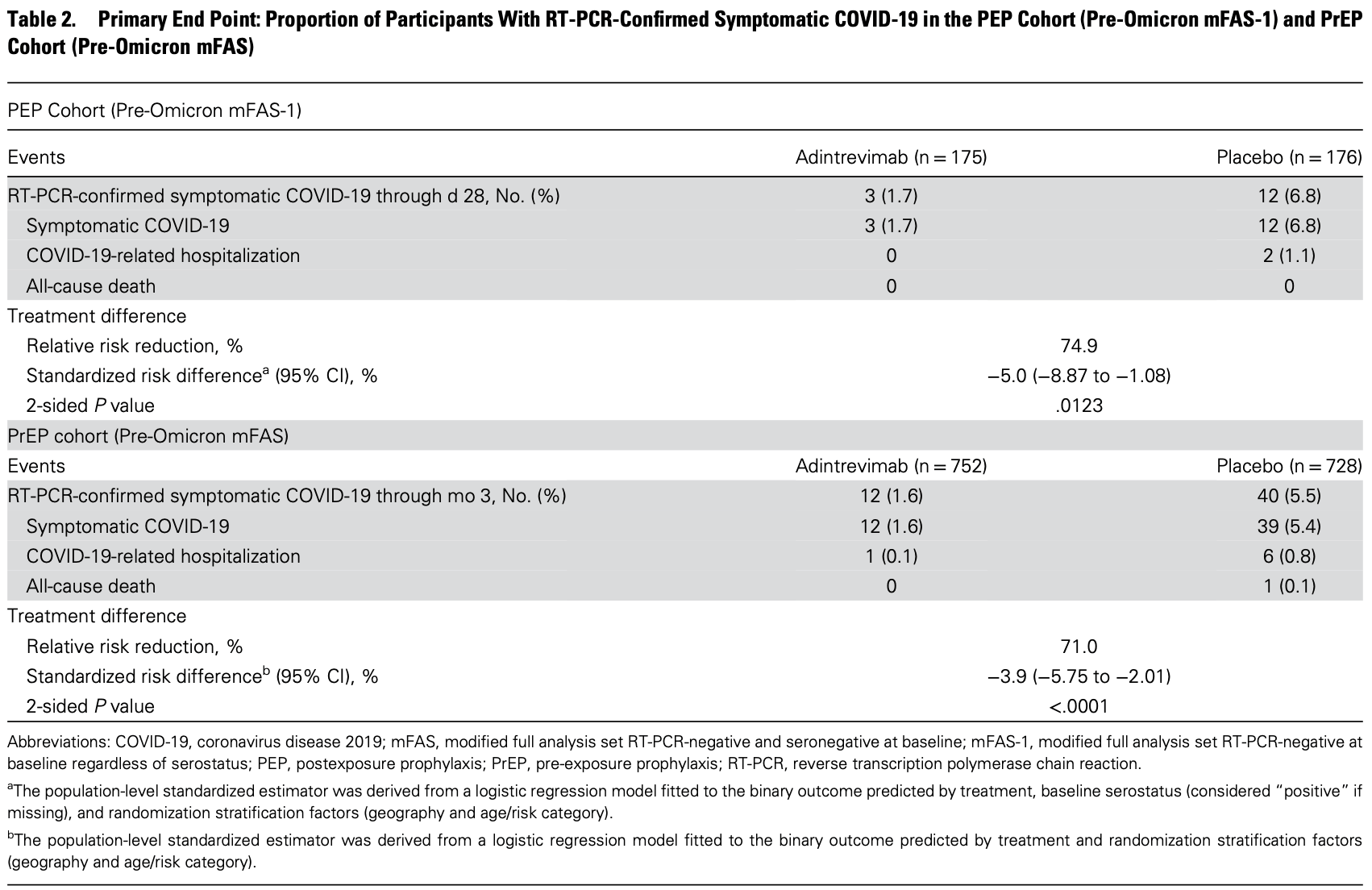
PrEP RCT 1480 patients, showing lower COVID-19 cases with adintrevimab. The PEP trial is listed separately1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 33.3% lower, RR 0.67, p = 1.00, treatment 2 of 1,001 (0.2%), control 3 of 1,001 (0.3%), NNT 1001, COVID-19, 6 months, supplementary table 6.
|
|
risk of death, 50.0% lower, RR 0.50, p = 0.51, treatment 3 of 1,001 (0.3%), control 6 of 1,001 (0.6%), NNT 334, all-cause, 6 months, supplementary table 6.
|
|
risk of death, 83.9% lower, RR 0.16, p = 0.07, treatment 1 of 752 (0.1%), control 6 of 728 (0.8%), NNT 145, 3 months.
|
|
risk of hospitalization, 83.9% lower, RR 0.16, p = 0.07, treatment 1 of 752 (0.1%), control 6 of 728 (0.8%), NNT 145, 3 months.
|
|
risk of symptomatic case, 70.2% lower, RR 0.30, p < 0.001, treatment 12 of 752 (1.6%), control 39 of 728 (5.4%), NNT 27, 3 months.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ison et al., 13 Jun 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 15 authors, study period 27 April, 2021 - 11 January, 2022, trial NCT04859517 (history) (EVADE PrEP).
Contact: pschmidt@invivyd.com.
Prevention of COVID-19 Following a Single Intramuscular Administration of Adintrevimab: Results From a Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial (EVADE)
Open Forum Infectious Diseases, doi:10.1093/ofid/ofad314
Background. The prevention of coronavirus disease 2019 in vulnerable populations is a global health priority. EVADE was a phase 2/3 multicenter, double-blind, randomized, placebo-controlled trial of adintrevimab, an extended-half-life monoclonal antibody, for postexposure (PEP) and pre-exposure prophylaxis (PrEP) of symptomatic COVID-19. Methods. Eligible participants (vaccine-naive, aged ≥12 years) were randomized 1:1 to receive a single 300-mg intramuscular injection of adintrevimab or placebo. Primary efficacy end points were reverse transcription polymerase chain reaction (RT-PCR)confirmed symptomatic COVID-19 through day 28 in the PEP cohort (RT-PCR-negative at baseline) and through month 3 in the PrEP cohort (RT-PCR-negative and seronegative at baseline) among participants randomized before emergence of the severe acute respiratory syndrome coronavirus 2 Omicron variant (November 30, 2021). Safety was assessed through 6 months. Results. Between April 27, 2021, and January 11, 2022, 2582 participants were randomized. In the primary efficacy analysis, RT-PCR-confirmed symptomatic COVID-19 occurred in 3/175 (1.7%) vs 12/176 (6.8%) adintrevimab-and placebo-treated PEP participants, respectively (74.9% relative risk reduction [RRR]; standardized risk difference, -5.0%; 95% CI, -8.87% to -1.08%; P = .0123) and in 12/752 (1.6%) vs 40/728 (5.5%) adintrevimab-and placebo-treated PrEP participants, respectively (71.0% RRR; standardized risk difference, -3.9%; 95% CI, -5.75% to -2.01%; P < .0001). In a prespecified exploratory analysis of 428 PrEP participants randomized after the emergence of Omicron, adintrevimab reduced RT-PCR-confirmed symptomatic COVID-19 by 40.6% (standardized risk difference -8.4%; 95% CI, -15.35% to -1.46%; nominal P = .0177) vs placebo. Adintrevimab was well tolerated, with no serious drug-related adverse events reported. Conclusions. A single intramuscular injection of adintrevimab provided prophylactic efficacy against COVID-19 due to susceptible variants without safety concerns. Clinical trial registration. NCT04859517.
Supplementary Data Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author. Author contributions. L.E.C., A.H., A.P., and K.N. contributed to study design. L.E.C., A.H., A.P., and K.N. were involved in protocol development. D.W. and M.D. were principal investigators. P.S. provided oversight and leadership responsibility for the research activity planning and execution. A.H. and P.S. were responsible for medical monitoring. All authors contributed to data interpretation and were involved in drafting and critically revising the manuscript, and all authors approved the final version and are accountable for the accuracy and integrity of the manuscript. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication. Y.L. and D.G. verified the data. Financial support. This work was supported by Invivyd, Inc. (formerly Adagio Therapeutics, Inc.). Potential conflicts of interest. At the time of the study, M.G.I. received research support, paid to Northwestern University, from GlaxoSmithKline; received payment for consultation from ADMA Biologics, AlloVir, Atea, Cidara, Genentech, Invivyd, Roche, Janssen, Shionogi, Takeda, and Viracor Eurofins; received royalties from UpToDate;..
References
Altmann, Boyton, COVID-19 vaccination: the road ahead, Science
Augustin, Schommers, Stecher, Post-COVID syndrome in nonhospitalised patients with COVID-19: a longitudinal prospective cohort study, Lancet Reg Health Eur
Center, None
Collie, Nayager, Bamford, Bekker, Zylstra et al., Effectiveness and durability of the BNT162b2 vaccine against Omicron sublineages in South Africa, N Engl J Med
Corti, Purcell, Snell, Veesler, Tackling COVID-19 with neutralizing monoclonal antibodies, Cell
Cutler, The costs of long COVID, JAMA Health Forum
Cutler, The economic cost of long COVID: an update
Dejnirattisai, Huo, Zhou, SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses, Cell
Dejnirattisai, Zhou, Supasa, Antibody evasion by the P.1 strain of SARS-CoV-2, Cell
Earnest, Uddin, Matluk, Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. Cell Rep Med
Feikin, Higdon, Abu-Raddad, Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression, Lancet
Fiolet, Kherabi, Macdonald, Ghosn, Peiffer-Smadja, Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review, Clin Microbiol Infect
Focosi, Casadevall, A critical analysis of the use of cilgavimab plus tixagevimab monoclonal antibody cocktail (Evusheld) for COVID-19 prophylaxis and treatment, Viruses
Ge, Durham, Meyer, Xie, Thomas, Covariate-adjusted difference in proportions from clinical trials using logistic regression and weighted risk differences, Drug Inf J
Herman, Brien, Forleo-Neto, Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis
Ison, Popejoy, Evgeniev, Efficacy and safety of adintrevimab (ADG20) for the treatment of high-risk ambulatory patients with mild or moderate COVID-19: results from a phase 2/3, randomized, placebo-controlled trial (STAMP) conducted during Delta predominance and early emergence of Omicron, Open Forum Infect Dis
Jondreville, 'aveni, Wallet, Pre-exposure prophylaxis with tixagevimab/cilgavimab (AZD7442) prevents severe SARS-CoV-2 infection in recipients of allogeneic hematopoietic stem cell transplantation during the Omicron wave: a multicentric retrospective study of SFGM-TC, J Hematol Oncol
Jurdi, Morena, Cote, Bethea, Azzi et al., Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the Omicron wave, Am J Transplant
Kaku, Narayan, Schmidt, Engler, Li et al., ADG20, a half-life-extended monoclonal antibody in development for the prevention and treatment of COVID-19, demonstrated broad in vitro neutralisation against SARS-CoV-2 variants. Paper presented at: 32nd European Congress of Clinical Microbiology & Infectious Diseases
Lee, Wong, Chai, Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ
Levin, Ustianowski, Thomas, AZD7442 (tixagevimab/cilgavimab) for post-exposure prophylaxis of symptomatic coronavirus disease, Clin Infect Dis
Levin, Ustianowski, Wit, Intramuscular AZD7442 (tixagevimabcilgavimab) for prevention of COVID-19, N Engl J Med
Liu, Ginn, Dejnirattisai, Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum, Cell
Marovich, Mascola, Cohen, Monoclonal antibodies for prevention and treatment of COVID-19, JAMA
Moon, Brown, Rosenthal, Healthcare resource utilization of patients with COVID-19 visiting US hospitals, Value Health
Nguyen, Flahault, Chavarot, Pre-exposure prophylaxis with tixagevimab and cilgavimab (evusheld) for COVID-19 among 1112 severely immunocompromised patients, Clin Microbiol Infect
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent COVID-19, N Engl J Med
Rappazzo, Tse, Kaku, Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody, Science
Rubino, Ambrose, Connolly, Pu, Population pharmacokinetics of ADG20, an extended-half-life monoclonal antibody being developed for the treatment and prevention of COVID-19
Schmidt, Gong, Narayan, Safety, pharmacokinetics, serum neutralizing titers, and immunogenicity of adintrevimab, a monoclonal antibody targeting SARS-CoV-2: a randomized, double-blind, placebo-controlled, phase 1 dose-escalation study in healthy adults, Infect Dis Ther
Schmidt, Narayan, Li, Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers, Sci Transl Med
Shiels, Haque, De González, Freedman, Leading causes of death in the US during the COVID-19 pandemic, JAMA Intern Med
Totschnig, Niculescu, SARS-CoV-2 pre-exposure prophylaxis with sotrovimab and tixagevimab/cilgavimab in immunocompromised patients-a single-center experience, Viruses
Twohig, Nyberg, Zaidi, Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study, Lancet Infect Dis
Van Wart, Tarbell, Walker, Connolly, Ambrose, Use of a whole-body quantitative system pharmacology physiologically based pharmacokinetic model to support ADG20 dose selection for the prevention of coronavirus disease (COVID-19)
Walker, Pu, Gong, Hawn, Schmidt, Validation of a predictive model to correlate neutralization titers and efficacy for the prevention of COVID-19
Wec, Wrapp, Herbert, Broad neutralization of SARS-related viruses by human monoclonal antibodies, Science
West, Wec, Doyle, Paper presented at: 33rd European Congress of Clinical Microbiology and Infectious Diseases
Xie, Bowe, Al-Aly, Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status, Nat Commun
Zumbrun, Kaku, Dillinger, Prophylactic administration of the monoclonal antibody adintrevimab protects against SARS-CoV-2 in hamster and non-human primate models of COVID-19, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1093/ofid/ofad314",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofad314",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The prevention of COVID-19 in vulnerable populations is a global health priority. EVADE was a phase 2/3 multicenter, double-blind, randomized, placebo-controlled trial of adintrevimab, an extended–half-life monoclonal antibody, for post-exposure (PEP) and pre-exposure prophylaxis (PrEP) of symptomatic COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Eligible participants (vaccine-naïve, aged ≥12 years) were randomized 1:1 to receive a single 300-mg intramuscular injection of adintrevimab or placebo. Primary efficacy endpoints were reverse-transcription polymerase chain reaction (RT-PCR)-confirmed symptomatic COVID-19 through day 28 in the PEP cohort (RT-PCR-negative at baseline) and through month 3 in the PrEP cohort (RT-PCR-negative and seronegative at baseline) among participants randomized before emergence of the SARS-CoV-2 Omicron variant (30 November 2021). Safety was assessed through 6 months.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Between 27 April 2021, and 11 January 2022, 2582 participants were randomized. In the primary efficacy analysis, RT-PCR-confirmed symptomatic COVID-19 occurred in 3/175 (1.7%) vs 12/176 (6.8%) adintrevimab- and placebo-treated PEP participants, respectively (74.9% relative risk reduction [RRR]; standardized risk difference –5.0%; 95% CI, –8.87 to –1.08; P=.0123), and in 12/752 (1.6%) vs 40/728 (5.5%) adintrevimab- and placebo-treated PrEP participants, respectively (71.0% RRR; standardized risk difference –3.9%; 95% CI, –5.75 to –2.01; P&lt;.0001). In a prespecified exploratory analysis of 428 PrEP participants randomized after emergence of Omicron, adintrevimab reduced RT-PCR-confirmed symptomatic COVID-19 by 40.6% (standardized risk difference −8.4%; 95% CI, −15.35 to −1.46; nominal P=.0177) vs placebo. Adintrevimab was well tolerated with no serious drug-related adverse events reported.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>A single intramuscular injection of adintrevimab provided prophylactic efficacy against COVID-19 due to susceptible variants without safety concerns.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Respiratory Diseases Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases , Rockville, Maryland , USA"
}
],
"family": "Ison",
"given": "Michael G",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Science 37 Inc. , Culver City, California , USA"
}
],
"family": "Weinstein",
"given": "Debra F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kyiv City Clinical Hospital No. 12, Department of Emergency Care and ARENSIA Exploratory Medicine , Kyiv , Ukraine"
}
],
"family": "Dobryanska",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Holmes",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Phelan",
"given": "Anne-Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Li",
"given": "Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Gupta",
"given": "Deepali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Narayan",
"given": "Kristin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Tosh",
"given": "Kazima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Hershberger",
"given": "Ellie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Connolly",
"given": "Lynn E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Yalcin",
"given": "Ilker",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Campanaro",
"given": "Ed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Hawn",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Invivyd, Inc. , Waltham, Massachusetts , USA"
}
],
"family": "Schmidt",
"given": "Pete",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the EVADE Study Group",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T20:56:39Z",
"timestamp": 1686689799000
},
"deposited": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T20:56:39Z",
"timestamp": 1686689799000
},
"indexed": {
"date-parts": [
[
2023,
6,
14
]
],
"date-time": "2023-06-14T04:18:30Z",
"timestamp": 1686716310667
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
6,
13
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
13
]
],
"date-time": "2023-06-13T00:00:00Z",
"timestamp": 1686614400000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofad314/50598666/ofad314.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofad314/50598666/ofad314.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
6,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
13
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofad314/7197273"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "Prevention of COVID-19 Following a Single Intramuscular Administration of Adintrevimab: Results From a Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial (EVADE)",
"type": "journal-article"
}