Balancing data quality and participant burden: A comparative analysis of abbreviated vs extended symptom diaries in the CanTreatCOVID trial
et al., medRxiv, doi:10.64898/2026.01.23.26343617, Jan 2026
Secondary analysis of the CanTreatCOVID paxlovid RCT comparing adherence and symptom reporting between a 9-item abbreviated diary and 34-item FLU-PRO Plus diary in 712 COVID-19 outpatients, showing no significant difference in compliance, with low compliance in both groups (68.1% vs 64.4%). Predictive models for recovery showed only marginal gains from the extended 34-item format. Authors conclude that abbreviated symptom diaries provide comparable data quality while potentially reducing participant burden.
Patients in the treatment group were significantly more likely to report data in this open-label trial, and the analysis provides information on the potential impact of missing data in these kind of trials.
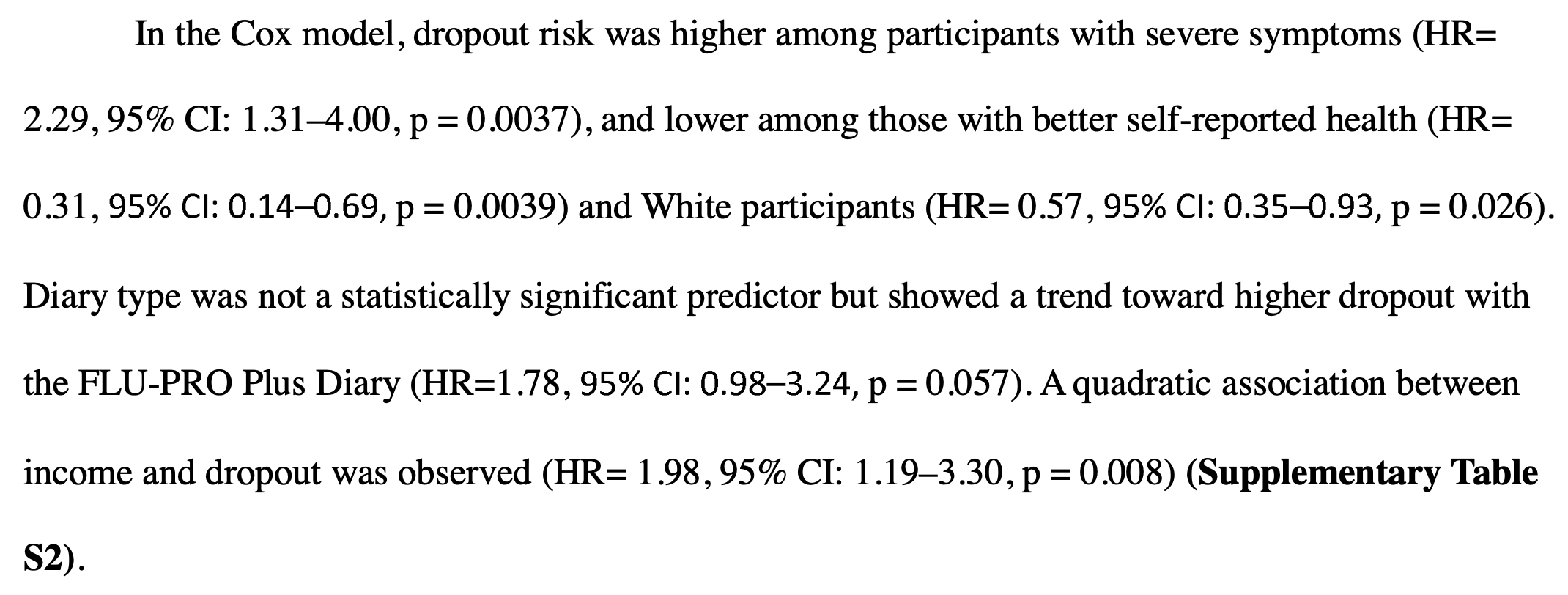
Patients with severe symptoms and worse self-reported health were more likely to not report data. Combined with the group differential where control patients were more likely to not report data, this means that efficacy may be underestimated when not accounting for missing data (since more of the more severe control patients are missing).
Authors have released this secondary analysis but have not released the results of the study for the efficacy of paxlovid.
Hosseini et al., 28 Jan 2026, Randomized Controlled Trial, Canada, preprint, 15 authors.
Contact: andrew.pinto@utoronto.ca.
Balancing data quality and participant burden: A comparative analysis of abbreviated vs extended symptom diaries in the CanTreatCOVID trial
doi:10.64898/2026.01.23.26343617
Background: Symptom diaries are widely used in acute respiratory infection trials to capture patientreported symptom severity and recovery. Longer questionnaires may provide a more complete clinical picture but can increase participant burden and reduce adherence. Evidence directly comparing long and short formats within the same trial is limited. Objective: To compare adherence, symptom trajectories, agreement between recovery measures, and predictive performance for recovery-related outcomes between a short and long symptom diary in an outpatient SARS-CoV-2 trial Methods: This secondary analysis of the CanTreatCOVID trial compared a 9-item Abbreviated Diary and a 34-item FLU-PRO Plus Diary over 14 days in non-hospitalized participants with confirmed SARS-CoV-2 infection. Outcomes included diary initiation, completion, completion rate, compliance, symptom trajectories, agreement between recovery outcomes, and predictive performance. Analyses used logistic regression, generalized estimating equations, survival models, and predictive modelling.
References
Butler, Dorward, Yu, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Fraia, Tripodi, Arasi, Adherence to Prescribed E-Diary Recording by Patients With Seasonal Allergic Rhinitis: Observational Study, J Med Internet Res
Frost, Mcclurg, Brady, Williams, Optimising the validity and completion of adherence diaries: a multiple case study and randomised crossover trial, Trials
Han, Poon, Powers, Leidy, Yu et al., Using the Influenza Patient-reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model, BMC Infect Dis
Hayward, Butler, Yu, Platform Randomised trial of INterventions against COVID-19 In older peoPLE (PRINCIPLE): protocol for a randomised, controlled, open-label, adaptive platform, trial of community treatment of COVID-19 syndromic illness in people at higher risk, BMJ Open
Hosseini, Condon, Da Costa, Canadian Adaptive Platform Trial of Treatments for COVID in Community Settings (CanTreatCOVID): protocol for a randomised controlled adaptive platform trial of treatments for acute SARS-CoV-2 infection in community settings, BMJ Open
Kato, Miura, The impact of questionnaire length on the accuracy rate of online surveys, Journal of Marketing Analytics
Kost, Da Rosa, Impact of survey length and compensation on validity, reliability, and sample characteristics for Ultrashort-, Short-, and Long-Research Participant Perception Surveys, J Clin Transl Sci
Langer, Klee, Gottschick, Mikolajczyk, Birth cohort studies using symptom diaries for assessing respiratory diseases-a scoping review, PLoS One
Lee, Morris, Grover, Murthy, Mcdonald, Outpatient Therapies for COVID-19: How Do We Choose?, Open Forum Infect Dis
Mccambridge, Kalaitzaki, White, Impact of Length or Relevance of Questionnaires on Attrition in Online Trials: Randomized Controlled Trial, J Med Internet Res
Powers, Bacci, Guerrero, Reliability, Validity, and Responsiveness of InFLUenza Patient-Reported Outcome (FLU-PRO©) Scores in Influenza-Positive Patients, Value in Health
Powers, Guerrero, Leidy, Development of the Flu-PRO: a patient-reported outcome (PRO) instrument to evaluate symptoms of influenza, BMC Infect Dis
Richard, Epsi, Pollett, Performance of the inFLUenza Patient-Reported Outcome Plus (FLU-PRO Plus) Instrument in Patients With Coronavirus Disease 2019, Open Forum Infect Dis
Rolstad, Adler, Rydén, Response Burden and Questionnaire Length: Is Shorter Better? A Review and Meta-analysis, Value in Health
Schneider, Stone, Ambulatory and diary methods can facilitate the measurement of patient-reported outcomes, Quality of Life Research
Stone, Shiffman, Schwartz, Broderick, Hufford, Patient compliance with paper and electronic diaries, Control Clin Trials
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, openlabel, adaptive platform trial, The Lancet
DOI record:
{
"DOI": "10.64898/2026.01.23.26343617",
"URL": "http://dx.doi.org/10.64898/2026.01.23.26343617",
"abstract": "<jats:p>Background: Symptom diaries are widely used in acute respiratory infection trials to capture patient-reported symptom severity and recovery. Longer questionnaires may provide a more complete clinical picture but can increase participant burden and reduce adherence. Evidence directly comparing long and short formats within the same trial is limited. \n\nObjective: To compare adherence, symptom trajectories, agreement between recovery measures, and predictive performance for recovery-related outcomes between a short and long symptom diary in an outpatient SARS-CoV-2 trial.\n\nMethods: This secondary analysis of the CanTreatCOVID trial compared a 9-item Abbreviated Diary and a 34-item FLU-PRO Plus Diary over 14 days in non-hospitalized participants with confirmed SARS-CoV-2 infection. Outcomes included diary initiation, completion, completion rate, compliance, symptom trajectories, agreement between recovery outcomes, and predictive performance. Analyses used logistic regression, generalized estimating equations, survival models, and predictive modelling. \n\nResults: Of 712 participants, 638 used the Abbreviated Diary and 74 the FLU-PRO Plus Diary. Baseline characteristics were similar between groups. Diary type was not significantly associated with diary initiation or full 14-day compliance, whereas treatment assignment was associated with higher adherence (p < 0.0001). Completion rates were slightly higher in the Abbreviated group (68.1% vs. 64.4%), but differences were not statistically significant. Agreement between \"feeling recovered\" and \"return to usual health\" was strong to excellent (κ = 0.8371-0.8859), while agreement with \"return to usual activities\" was moderate (κ = 0.5273-0.6583). Predictive models performed well for both diaries (AUCs 0.87-0.94), with only marginal gains from including the extended FLU-PRO Plus items. \n\nConclusion: In this outpatient SARS-CoV-2 trial, abbreviated and extended symptom diaries produced comparable adherence, symptom trajectories, and predictive performance. Future research should extend follow-up beyond 14 days to capture longer-term patterns and test diary performance in more diverse and digitally underserved populations.</jats:p>",
"accepted": {
"date-parts": [
[
2026,
1,
28
]
]
},
"author": [
{
"affiliation": [
{
"name": "Upstream Lab, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada; Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada;"
}
],
"family": "Hosseini",
"given": "Banafshe (Benita)",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Upstream Lab, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada;"
}
],
"family": "Mohammadrezaei",
"given": "Dorsa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Upstream Lab, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada;"
}
],
"family": "Balalaie",
"given": "Parsa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Upstream Lab, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, Unity Health Toronto, Toronto, Ontario, Canada;"
}
],
"family": "Sivayoganathan",
"given": "Kawsika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine, Max Rady College of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada;"
}
],
"family": "Condon",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK; Institute of Health Policy, Management and Evaluation, Dalla Lana School of Public Health, University of Toronto, To;"
}
],
"family": "da Costa",
"given": "Bruno R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine, Memorial University of Newfoundland, St. John's, Newfoundland and Labrador, Canada; Eastern Regional Health Authority, St. John's, Newfoundland and Labrador, Canada.;"
}
],
"family": "Daley",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family and Community Medicine, University of Toronto, Toronto, Ontario, Canada; Department of Family and Community Medicine, North York General Hospital, Toronto, Ontario, Canada;"
}
],
"family": "Greiver",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Trial Service Unit and Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, UK; Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada;"
}
],
"family": "Jüni",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Faculty of Medicine and Health Sciences, McGill University, Montreal, Quebec, Canada;"
}
],
"family": "Lee",
"given": "Todd C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Family Medicine, University of Calgary Cumming School of Medicine, Calgary, Alberta, Canada; Department Community Health Sciences, University of Calgary, Calgary, Alberta, Canada;"
}
],
"family": "McBrien",
"given": "Kerry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Experimental Medicine, Department of Medicine, McGill University, Montreal, Quebec, Canada; Division of General Internal Medicine, Department of Medicine, McGill University, Montreal, Quebec, Canada;"
}
],
"family": "McDonald",
"given": "Emily G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, Faculty of Medicine, University of British Columbia, Vancouver, British Columbia, Canada;"
}
],
"family": "Murthy",
"given": "Srinivas C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Addiction Programs, Centre for Addiction and Mental Health, Toronto, Ontario, Canada;"
}
],
"family": "Selby",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Upstream Lab, MAP, Li Ka Shing Knowledge Institute, Unity Health Toronto"
}
],
"family": "Pinto",
"given": "Andrew D",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
29
]
],
"date-time": "2026-01-29T06:15:18Z",
"timestamp": 1769667318000
},
"deposited": {
"date-parts": [
[
2026,
1,
29
]
],
"date-time": "2026-01-29T06:15:18Z",
"timestamp": 1769667318000
},
"group-title": "Health Systems and Quality Improvement",
"indexed": {
"date-parts": [
[
2026,
1,
30
]
],
"date-time": "2026-01-30T08:10:58Z",
"timestamp": 1769760658540,
"version": "3.49.0"
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2026,
1,
28
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
28
]
],
"date-time": "2026-01-28T00:00:00Z",
"timestamp": 1769558400000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.64898/2026.01.23.26343617",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "54368",
"original-title": [],
"posted": {
"date-parts": [
[
2026,
1,
28
]
]
},
"prefix": "10.64898",
"published": {
"date-parts": [
[
2026,
1,
28
]
]
},
"publisher": "openRxiv",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.64898/2026.01.23.26343617"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Balancing data quality and participant burden: A comparative analysis of abbreviated vs extended symptom diaries in the CanTreatCOVID trial",
"type": "posted-content"
}