The prophylactic efficacy of Anatolian propolis in individuals at high risk of COVID-19
et al., European Review for Medical and Pharmacological Sciences, doi:10.26355/eurrev_202212_30483, NCT04680819, Oct 2021 (preprint)
Prospective study of 204 high-risk healthcare professionals showing significantly lower COVID-19 infection with prophylactic use of Anatolian propolis drops over one month. The treatment group received 20 drops of 30% Anatolian propolis extract twice daily.
Figure 1 indicates randomization, however the text indicates an observational study with participants equally divided between groups, and the registry indicates that the control group was to be from people that do not agree to participate.
|
risk of case, 85.7% lower, RR 0.14, p = 0.003, treatment 2 of 102 (2.0%), control 14 of 102 (13.7%), NNT 8.5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Guler et al., 21 Oct 2021, prospective, Turkey, peer-reviewed, mean age 35.2, 5 authors, study period 25 December, 2020 - 25 January, 2021, trial NCT04680819 (history).
in individuals at high risk of COVID-19
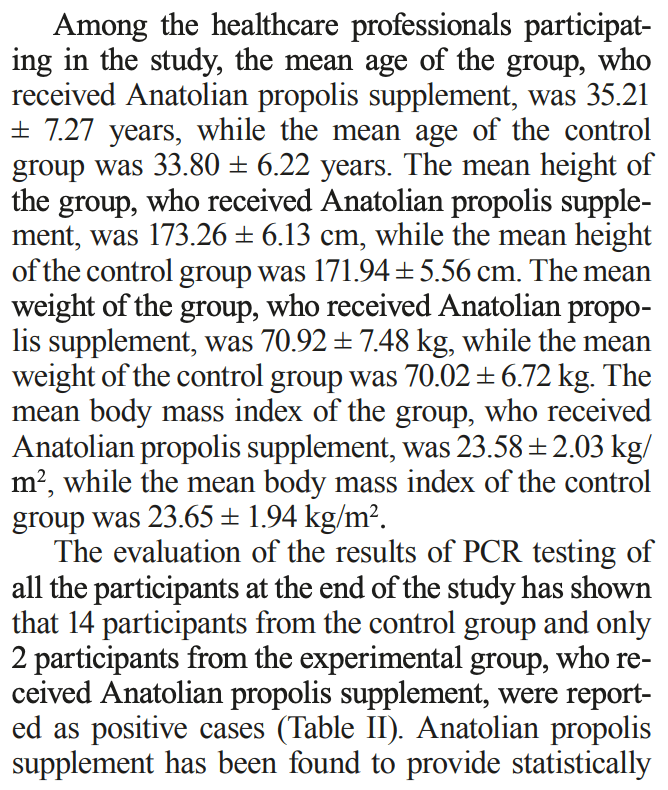
OBJECTIVE: No prophylactic treatment is available for individuals at high risk of developing COVID-19. This study, which was conducted between December 25, 2020, and January 25, 2021, is one of the first clinical studies to evaluate the efficacy of Anatolian propolis supplement against COVID-19. The aim was to obtain evidence on the prophylactic use of Anatolian propolis in individuals at high risk of developing COVID-19. SUBJECTS AND METHODS: This volunteer-based study was conducted in two centers. The study involved 209 healthcare professionals (physicians, nurses, medical secretaries) from Emergency Medicine Department of Medical Faculty of Ataturk University and Emergency Medicine Department of Rize Recep Tayyip Erdogan University. 204 participants meeting the study criteria were divided into two groups as experimental group and control group. The experimental group received 20 drops of BEE'O UP (BEE&YOU) 30% Propolis drops twice a day during a follow-up period of 1 month. The control group received no supplement but was followed up. The participants showing symptoms during the study and all the participants at the end the study were subjected to PCR testing. RESULTS: The evaluation of the results of PCR testing at the end of the study has shown that 14 participants from the control group and only 2 participants from the experimental group, who received Anatolian propolis supplement, were reported as positive cases. CONCLUSIONS: It has been found that a statistically significant protection was induced against COVID-19 infection in 98% of the experimental group, who received Anatolian propolis, compared to the control group.
Authors' Contributions Authors equally contributed to planning, experiment, writing the manuscript.
Conflict of Interest Aslı Elif Tanugur Samanci is the Scientific Director of SBS Scientific Bio Solutions Bee&You R&D Center, Istanbul, Turkey.
Informed Consent Patients participated in the study voluntarily and written consent was obtained to have these data.
Ethics Approval The patients who will participate in the study and the documents obtained after the study were examined and approved by the Atatürk University Faculty of Medicine Ethics Committee. This committee has provided prospective ethical approval for this study.
ORCID ID Enes Güler: 0000-0003-4926-0332 Ozlem Bilir: 0000-0001-9016-1665 Abdullah Osman Kocak: 0000-0002-1678-4474 İsmail Atas: 0000-0001-6723-8564 Aslı Elif Tanugur Samanci: 0000-0003-1639-6495
References
Bachevski, Damevska, Simeonovski, Dimova, Back to the basics: Propolis and COVID-19, Dermatol Ther
Berretta, Silveira, Capcha, Jong, Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease, Biomed Pharmacother
Braakhuis, Evidence on the health benefits of supplemental propolis, Nutrients
Buha, Mirić, Agić, Simić, Stjepanović et al., A randomized, double-blind, placebo-controlled study evaluating the efficacy of propolis and N-acetylcysteine in exacerbations of chronic obstructive pulmonary disease, Eur Rev Med Pharmacol Sci
Daziroğlu, Yildiz, Akbulut, COVID-19 Pandemisine Diyetetik Bakış: Besin, Besin Destekleri ve Tıbbi Beslenme Tedavisi, ERÜ Sağlık Bilimleri Fakültesi Dergisi
Esposito, Garzarella, Bocchino, 'avino, Caruso et al., A standardized polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): A monocentric, randomized, double-blind, placebo-controlled clinical trial, Phytomedicine
Guler, Tatar, Yildiz, Belduz, Kolayli, Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study, Arch Microbiol
Güler, Şal, Can, Kara, Yildiz et al., Targeting CoV-2 spike RBD and ACE-2 interaction with flavonoids of Anatolian propolis by in silico and in vitro studies in terms of possible COVID-19 therapeutics, Turk J Biol
Hashem, In Silico approach of some selected honey constituents as SARS-CoV-2 main protease (COVID-19) inhibitors, EJMO
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Khalil, Tazeddinova, The upshot of Polyphenolic compounds on immunity amid COVID-19 pandemic and other emerging communicable diseases: An appraisal, Nat Prod Bioprospect
Kosari, Noureddini, Khamechi, Najafi, Ghaderi et al., The effect of propolis plus Hyoscyamus niger L. methanolic extract on clinical symptoms in patients with acute respiratory syndrome suspected to COVID-19: A clinical trial, Phytother Res
Lima, Brito, Da, Nizer, Bee products as a source of promising therapeutic and chemoprophylaxis strategies against COVID-19 (SARS-CoV-2), Phytother Res
Maruta, He, PAK1-blockers: Potential Therapeutics against COVID-19, Med Drug Discov
Matteo, Spano, Grosso, Salvo, Ingallina et al., Food and COVID-19: Preventive/Co-therapeutic Strategies Explored by Current Clinical Trials and in Silico Studies, Foods
Mrityunjaya, Pavithra, Neelam, Janhavi, Halami et al., Immune-Boosting, Antioxidant and Anti-inflammatory Food Supplements Targeting Pathogenesis of COVID-19, Front Immunol
Mutlu, Uygun, Erden, Koronavirüs Hastalığı (COVID-19) Tedavisinde Kullanılan İlaçlar, Kocaeli Üniversitesi Sağlık Bilimleri Dergisi
Ripari, Sartori, Da, Honorio, Conte et al., Propolis antiviral and immunomodulatory activity: a review and perspectives for COVID-19 treatment, J Pharm Pharmacol
Russo, Moccia, Spagnuolo, Tedesco, Russo, Roles of flavonoids against coronavirus infection, Chem Biol Interact
Sağlık, Bakanlığı COVID-19 Bilgilendirme Platformu
Sharma, Tiwari, Deb, Marty, Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies, Int J Antimicrob Agents
Weng, Lin, Lai, Lin, Wang et al., Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63, Virus Res
Yasar, Aytekin, COVID-19 ve Beslenme Arasındaki İlişkiye Güncel Bir Bakış, Akademik Gıda
Zorlu, COVID-19 and Anatolian propolis: A case report, Acta Medica Mediterranea
DOI record:
{
"DOI": "10.26355/eurrev_202212_30483",
"ISSN": "1128-3602, 2284-0729",
"URL": "https://doi.org/10.26355/eurrev_202212_30483",
"abstract": "OBJECTIVE: No prophylactic treatment is available for individuals at high risk of developing COVID-19. This study, which was conducted between December 25, 2020, and January 25, 2021, is one of the first clinical studies to evaluate the efficacy of Anatolian propolis supplement against COVID-19. The aim was to obtain evidence on the prophylactic use of Anatolian propolis in individuals at high risk of developing COVID-19. SUBJECTS AND METHODS: This volunteer-based study was conducted in two centers. The study involved 209 healthcare professionals (physicians, nurses, medical secretaries) from Emergency Medicine Department of Medical Faculty of Ataturk University and Emergency Medicine Department of Rize Recep Tayyip Erdogan University. 204 participants meeting the study criteria were divided into two groups as experimental group and control group. The experimental group received 20 drops of BEE’O UP (BEE&YOU) 30% Propolis drops twice a day during a follow-up period of 1 month. The control group received no supplement but was followed up. The participants showing symptoms during the study and all the participants at the end the study were subjected to PCR testing. RESULTS: The evaluation of the results of PCR testing at the end of the study has shown that 14 participants from the control group and only 2 participants from the experimental group, who received Anatolian propolis supplement, were reported as positive cases. CONCLUSIONS: It has been found that a statistically significant protection was induced against COVID-19 infection in 98% of the experimental group, who received Anatolian propolis, compared to the control group.",
"author": [
{
"family": "Guler",
"given": "E."
},
{
"family": "Bilir",
"given": "O."
},
{
"family": "Osman Kocak",
"given": "A."
},
{
"family": "Atas",
"given": "I."
},
{
"family": "Tanugur Samanci",
"given": "A.E."
}
],
"container-title": "European Review for Medical and Pharmacological Sciences",
"issue": "2 Suppl",
"issued": {
"date-parts": [
[
2022,
12
]
]
},
"language": "eng",
"medium": "JB",
"page": "53-60",
"page-first": "53",
"publisher": "Verduci Editore s.r.l.",
"publisher-place": "IT",
"title": "The prophylactic efficacy of Anatolian propolis in individuals at high risk of COVID-19",
"type": "article-journal",
"volume": "26"
}