Emergence of SARS-CoV-2 Spike Escape Mutation Q493r After Bamlanivimab/Etesevimab Treatment for COVID-19
et al., Research Square, doi:10.21203/rs.3.rs-524959/v1, May 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
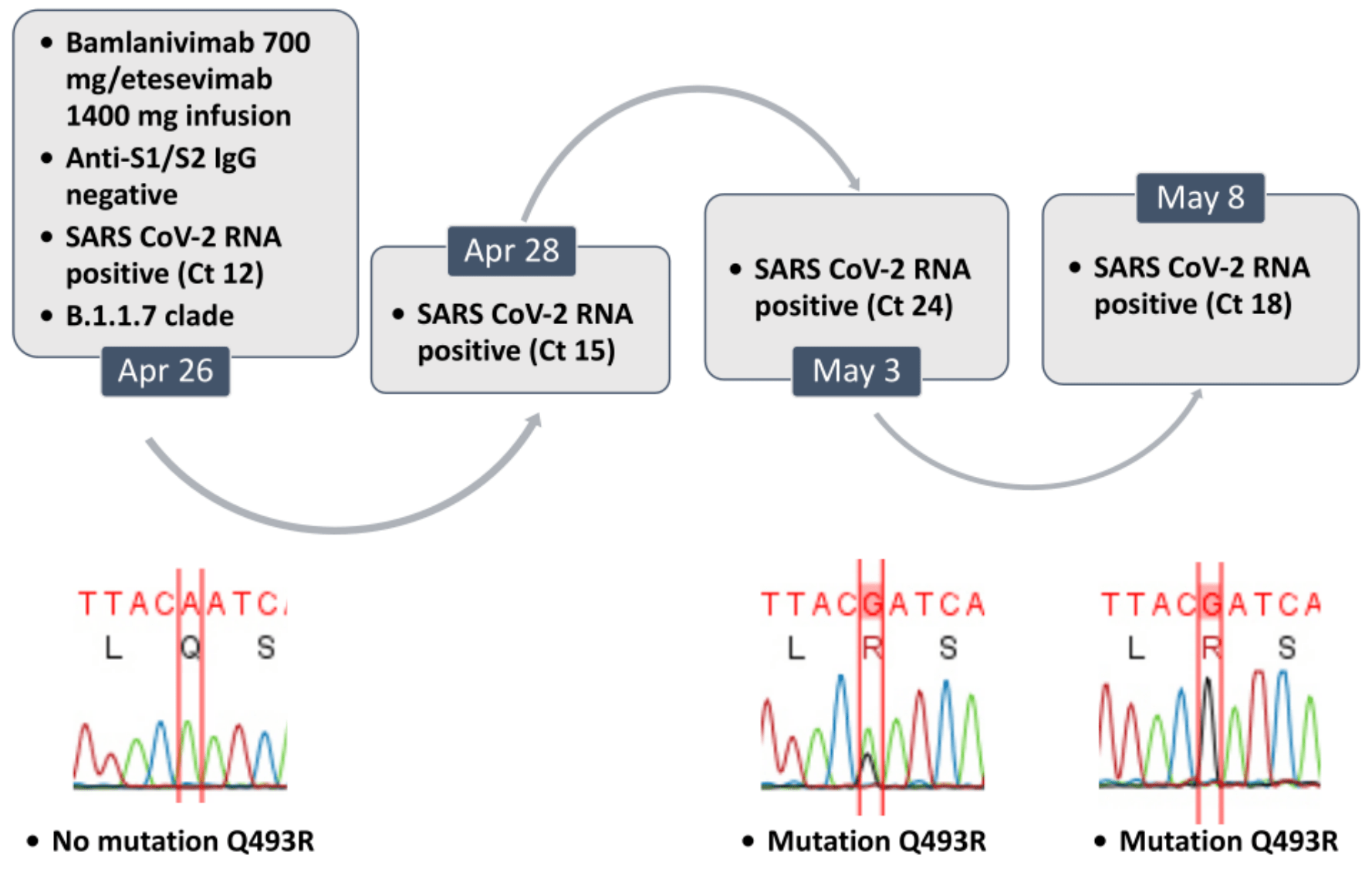
Case study showing that a mutation resistant to both bamlanivimab and etesevimab can arise in vivo. Authors note that accelerated evolution can occur under selective pressure from therapeutic interventions with neutralizing antibodies, and that bamlanivimab was withdrawn as a monotherapy because of failure against E484K-positive SARS-CoV-2 variants.
Focosi et al., 18 May 2021, preprint, 7 authors.
Emergence of SARS-CoV-2 Spike Escape Mutation Q493r After Bamlanivimab/Etesevimab Treatment for COVID-19
doi:10.21203/rs.3.rs-524959/v1
SARS-CoV-2 variants are usually a consequence of random mutations in humans or other hosts, but accelerated evolution can also occur under selective pressure from therapeutic interventions with neutralizing antibodies1. Bamlanivimab has been recently withdrawn from the vendor as a monotherapy because of failure against E484K-positive SARS-CoV-2 variants, but emergency use authorization remains in place for the bamlanivimab/etesevimab cocktail2, for which no completely resistant variant has been reported to date. We report here the rst in vivo case of a Spike escape mutation conferring combined resistance to both bamlanivimab and etesevimab.
Declarations We declare we have no con ict of interest related to this manuscript.
References
Chen, Zody, Mediavilla, Emergence of multiple SARS-CoV-2 antibody escape variants in an immunocompromised host undergoing convalescent plasma treatment
Ortega, Pujol, Jastrzebska, Rangel, Mutations in the SARS-CoV-2 spike protein modulate the virus a nity to the human ACE2 receptor, an in silico analysis, EXCLI journal
Starr, Greaney, Dingens, Bloom, Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016, Cel Reports Medicine
Weisblum, Schmidt, Zhang, Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants, eLife
DOI record:
{
"DOI": "10.21203/rs.3.rs-524959/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-524959/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>SARS-CoV-2 variants are usually a consequence of random mutations in humans or other hosts, but accelerated evolution can also occur under selective pressure from therapeutic interventions with neutralizing antibodies1. Bamlanivimab has been recently withdrawn from the vendor as a monotherapy because of failure against E484K-positive SARS-CoV-2 variants, but emergency use authorization remains in place for the bamlanivimab/etesevimab cocktail2, for which no completely resistant variant has been reported to date. We report here the first <jats:italic>in vivo</jats:italic> case of a Spike escape mutation conferring combined resistance to both bamlanivimab and etesevimab.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
5,
14
]
]
},
"author": [
{
"affiliation": [
{
"name": "North-Western Tuscany Blood Bank, Pisa University Hospital, Pisa, Italy"
}
],
"family": "Focosi",
"given": "Daniele",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Laboratory of Microbiology, ASST Sette Laghi, Varese, Italy"
}
],
"family": "Novazzi",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Surgery, University of Insubria, Varese, Italy"
}
],
"family": "Genoni",
"given": "Angelo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Surgery, University of Insubria, Varese, Italy"
}
],
"family": "Dentali",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Surgery, University of Insubria, Varese, Italy"
}
],
"family": "gasperina",
"given": "Daniela Dalla",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0088-2712",
"affiliation": [
{
"name": "Department of Medicine and Surgery, University of Insubria, Varese, Italy"
}
],
"authenticated-orcid": false,
"family": "Baj",
"given": "Andreina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine and Surgery, University of Insubria, Varese, Italy"
}
],
"family": "Maggi",
"given": "Fabrizio",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
5,
17
]
],
"date-time": "2021-05-17T13:39:52Z",
"timestamp": 1621258792000
},
"deposited": {
"date-parts": [
[
2022,
7,
29
]
],
"date-time": "2022-07-29T02:01:04Z",
"timestamp": 1659060064000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2024,
3,
3
]
],
"date-time": "2024-03-03T13:52:57Z",
"timestamp": 1709473977616
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2021,
5,
17
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
17
]
],
"date-time": "2021-05-17T00:00:00Z",
"timestamp": 1621209600000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-524959/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-524959/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
5,
17
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2021,
5,
17
]
]
},
"publisher": "Research Square Platform LLC",
"reference-count": 0,
"references-count": 0,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.3201/eid2710.211538",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-524959/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "Emergence of SARS-CoV-2 Spike Escape Mutation Q493r After Bamlanivimab/Etesevimab Treatment for COVID-19",
"type": "posted-content"
}
