Timing and Predictors of Loss of Infectivity Among Healthcare Workers With Mild Primary and Recurrent Coronavirus Disease 2019 (COVID-19): A Prospective Observational Cohort Study
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad535, Jun 2023 (preprint)
2nd treatment shown to increase risk in
November 2020, now with p = 0.00000029 from 27 studies, but still recommended in 103 countries.
6,400+ studies for
210+ treatments. c19early.org
|
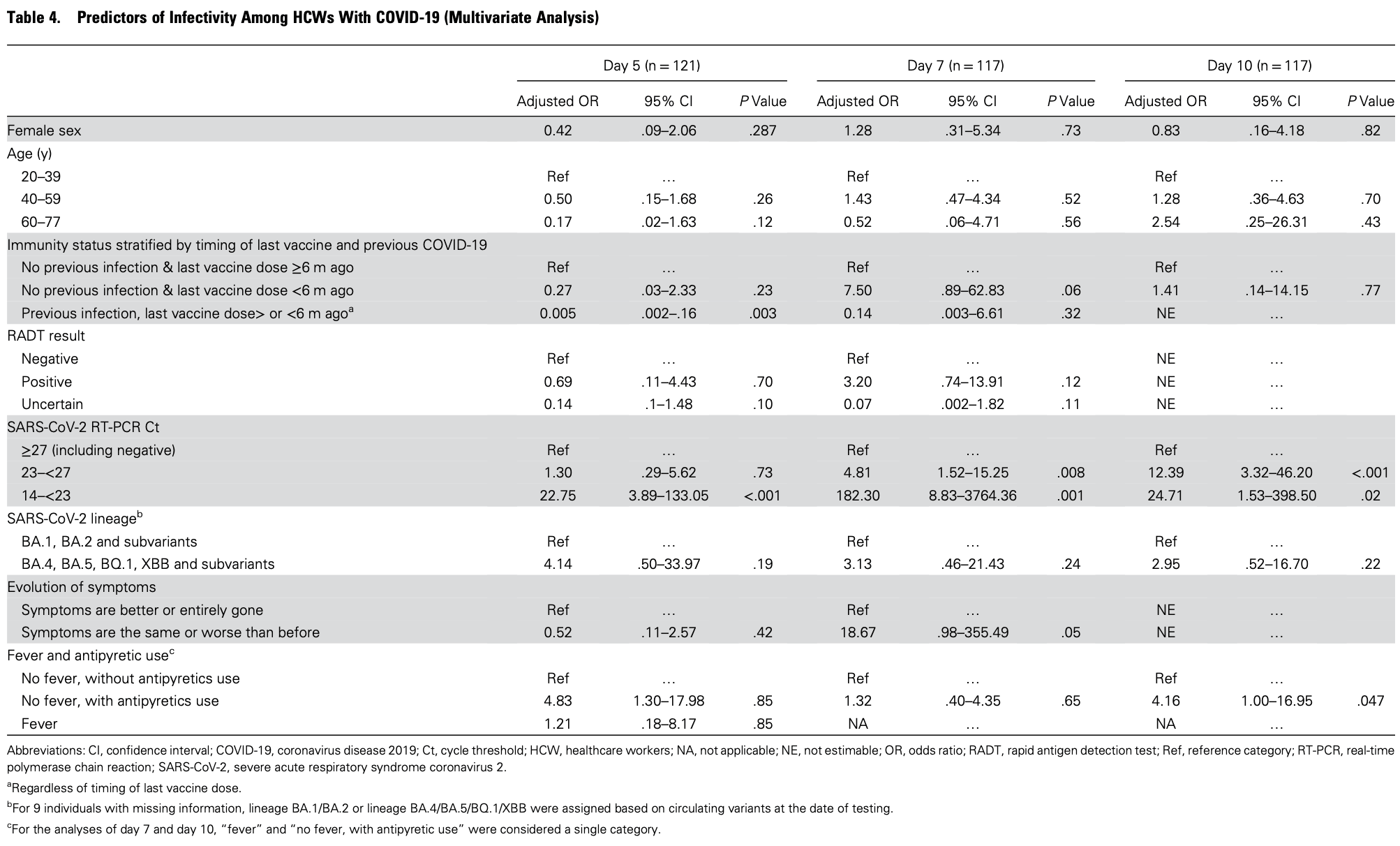
Prospective study of 121 healthcare workers with COVID-19, showing higher risk of viral infectivity with antipyretic use. The antipyretic medications are not specified. Day 5 results exclude the fever group, while the day 7 and day 10 results combine the fever group without specifying antipyretic use.
Acetaminophen is also known as paracetamol, Tylenol, Panadol, Calpol, Tempra, Calprofen, Doliprane, Efferalgan, Grippostad C, Dolo, Acamol, Fevadol, Crocin, and Perfalgan.
|
risk of no viral clearance, 383.0% higher, OR 4.83, p = 0.02, adjusted per study, multivariable, day 5, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Dzieciolowska et al., 18 Jun 2023, prospective, Canada, peer-reviewed, 14 authors.
Contact: yves.longtin@mcgill.ca.
Timing and Predictors of Loss of Infectivity among Healthcare Workers with Primary and Recurrent COVID-19: a Prospective Observational Cohort Study
doi:10.1101/2023.06.16.23291449
Background: There is a need to understand the duration of infectivity of primary and recurrent COVID-19 and identify predictors of loss of infectivity. Methods: Prospective observational cohort study with serial viral culture, rapid antigen detection test (RADT) and RT-PCR on nasopharyngeal specimens of healthcare workers with COVID-19. The primary outcome was viral culture positivity as indicative of infectivity. Predictors of loss of infectivity were determined using multivariate regression model. The performance of the US CDC criteria (fever resolution, symptom improvement and negative RADT) to predict loss of infectivity was also investigated. Results: 121 participants (91 female [79.3%]; average age, 40 years) were enrolled. Most (n=107, 88.4%) had received ≥3 SARS-CoV-2 vaccine doses, and 20 (16.5%) had COVID-19 previously. Viral culture positivity decreased from 71.9% (87/121) on day 5 of infection to 18.2% (22/121) on day 10. Participants with recurrent COVID-19 had a lower likelihood of infectivity than those with primary COVID-19 at each follow-up (day 5 OR, 0.14; p<0.001]; day 7 OR, 0.04; p=0.003]) and were all non-infective by day 10 (p=0.02). Independent predictors of infectivity included prior on day 5, 0.005; p=0.003), a RT-PCR Ct value <23 (aOR on day 5, 22.75; p<0.001), but not symptom improvement or RADT result. The CDC criteria would identify 36% (24/67) of all non-infectious individuals on Day 7. However, 17% (5/29) of those meeting all the criteria had a positive viral culture. Conclusions: Infectivity of recurrent COVID-19 is shorter than primary infections. Loss of infectivity algorithms could be optimized.
Coronavirus disease 2019 (COVID-19 ) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
1 The current evidence regarding duration of infectivity rely on viral culture to detect shedding of replication-competent virus (also called viable or infectious virus). These studies suggest that immunocompetent individuals with non-severe COVID-19 can remain infective for up to 10 days.
2-6 While infective, healthcare workers (HCWs) with COVID-19 must refrain from working to prevent nosocomial transmission.
7,8 However, the timing of their return to work is complicated by the interindividual variation in the durations of infectivity. Approximately a fifth of individuals may be infective for as little as 5 days, while approximately a quarter can remain infective for 10 days or more. 9 Determinants of loss of infectivity are largely unknown, but could be useful to optimize the return-towork of infected HCWs. To limit absenteeism, 10 the US Centers for Disease Control and Prevention (CDC) and European CDC have provided guidance to allow earlier return to work of eligible HCWs.
7,8 These algorithms use readily available information such as symptom improvement and the result of rapid antigen detection tests (RADT) to predict loss of infectivity.
7,8 However, whether these criteria can..
References
Aranha, Patel, Bhor, Gogoi, Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: An approach to reduce the isolation period for COVID-19 patients, J Med Virol, doi:10.1002/jmv.27206
Arons, Hatfield, Reddy, Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility, N Engl J Med, doi:10.1056/NEJMoa2008457
Bender, Lange, Adjusting for multiple testing--when and how?, J Clin Epidemiol. Apr, doi:10.1016/s0895-4356(00)00314-0
Black, Bailey, Przewrocka, Dijkstra, Swanton, COVID-19: the case for healthcare worker screening to prevent hospital transmission, Lancet, doi:10.1016/S0140-6736(20)30917-X11
Boucau, Marino, Regan, Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection, N Engl J Med, doi:10.1056/NEJMc2202092
Bouton, Atarere, Turcinovic, Viral Dynamics of Omicron and Delta Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants With Implications for Timing of Release from Isolation: A Longitudinal Cohort Study, Clin Infect Dis, doi:10.1093/cid/ciac510
Bullard, Dust, Funk, Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples, Clin Infect Dis, doi:10.1093/cid/ciaa638
Bullard, Dust, Funk, Predicting infectious SARS-CoV-2 from diagnostic samples, Clin Infect Dis, doi:10.1093/cid/ciaa638
Callow, Parry, Sergeant, Tyrrell, The time course of the immune response to experimental coronavirus infection of man, Epidemiol Infect. Oct, doi:10.1017/s0950268800048019
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe, doi:10.1016/S2666-5247(20)30172-5
Charest, Fafard, Carazo, Levade, Longtin et al., Statistical analyses: Carazo
Folgueira, Luczkowiak, Lasala, Perez-Rivilla, Delgado, Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19, Clin Microbiol Infect. Feb, doi:10.1016/j.cmi.2021.02.014
Gniazdowski, Morris, Wohl, Repeat COVID-19 Molecular Testing: Correlation of SARS-CoV-2 Culture with Molecular Assays and Cycle Thresholds, Clin Infect Dis, doi:10.1093/cid/ciaa1616
Gniazdowski, Morris, Wohl, Repeated Coronavirus Disease 2019 Molecular Testing: Correlation of Severe Acute Respiratory Syndrome Coronavirus 2 Culture With Molecular Assays and Cycle Thresholds, Clin Infect Dis, doi:10.1093/cid/ciaa1616
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control, J Clin Med. Apr, doi:10.3390/jcm9041225
Jefferson, Spencer, Brassey, Heneghan, Viral cultures for COVID-19 infectious potential assessment -a systematic review, Clin Infect Dis. Dec, doi:10.1093/cid/ciaa1764
Kadire, Fabre, Wenzel, Doctor, How Long Should I Isolate?, N Engl J Med, doi:10.1056/NEJMclde2100910
Keske, Esken, Vatansever, Duration of infectious shedding of SARS-CoV-2 omicron variant and its relation with symptoms, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.07.009
Keske, Guney-Esken, Vatansever, Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.07.009
Killingley, Mann, Kalinova, Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults, Nat Med, doi:10.1038/s41591-022-01780-9
Kim, Cui, Shin, Duration of Culturable SARS-CoV-2 in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMc2027040
Kissler, Hay, Fauver, Viral kinetics of sequential SARS-CoV-2 infections, doi:10.1101/2023.03.03.23286775
Landon, Bartlett, Marrs, Guenette, Weber et al., High Rates of Rapid Antigen Test Positivity After 5 days of Isolation for COVID-19
Lefferts, Blake, Bruden, Antigen Test Positivity After COVID-19 Isolation -Yukon-Kuskokwim Delta Region, Alaska, MMWR Morbidity and Mortality Weekly Report, doi:10.15585/mmwr.mm7108a3
Longtin, Charest, Quach, Infectivity of healthcare workers diagnosed with coronavirus disease 2019 (COVID-19) approximately 2 weeks after onset of symptoms: A cross-sectional study, Infect Control Hosp Epidemiol, doi:10.1017/ice.2020.1420
Longtin, Parkes, Charest, Persistence of infectivity in elderly individuals diagnosed with severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection 10 days after onset of symptoms: A cross-sectional study, Infect Control Hosp Epidemiol, doi:10.1017/ice.2021.502
O'toole, Scher, Underwood, Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool, Virus Evol, doi:10.1093/ve/veab064
Papenburg, Campbell, Caya, Adequacy of Serial Self-performed SARS-CoV-2 Rapid Antigen Detection Testing for Longitudinal Mass Screening in the Workplace, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.10559
Poon, Lin, Griffiths, Yong, Seah et al., A global overview of healthcare workers' turnover intention amid COVID-19 pandemic: a systematic review with future directions, Hum Resour Health, doi:10.1186/s12960-022-00764-7
Qi, Yang, Jiang, Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study, Int J Infect Dis, doi:10.1016/j.ijid.2020.05.045
Rothman, No adjustments are needed for multiple comparisons, Epidemiology. Jan
Sequencing, None
Stiefel, Bhullar, Zabarsky, Healthcare personnel frequently have positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen tests 5 days or more after diagnosis of coronavirus disease 2019 (COVID-19), Infection Control &, doi:10.1017/ice.2022.21
Takahashi, Ishikane, Ujiie, Duration of Infectious Virus Shedding by SARS-CoV-2 Omicron Variant-Infected Vaccinees. Emerg Infect Dis, doi:10.3201/eid2805.220197
Von Elm, Altman, Egger, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, J Clin Epidemiol. Apr, doi:10.1016/j.jclinepi.2007.11.008
Wolfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
Wu, Guo, Yuan, Duration of viable virus shedding and polymerase chain reaction positivity of the SARS-CoV-2 Omicron variant in the upper respiratory tract: a systematic review and meta-analysis, doi:10.1016/j.ijid.2023.02.011
Wurtz, Penant, Jardot, Duclos, Scola, Culture of SARS-CoV-2 in a panel of laboratory cell lines, permissivity, and differences in growth profile, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-020-04106-0
DOI record:
{
"DOI": "10.1093/cid/ciad535",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciad535",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>There is a need to understand the duration of infectivity of primary and recurrent coronavirus disease 2019 (COVID-19) and identify predictors of loss of infectivity.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Prospective observational cohort study with serial viral culture, rapid antigen detection test (RADT) and reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal specimens of healthcare workers with COVID-19. The primary outcome was viral culture positivity as indicative of infectivity. Predictors of loss of infectivity were determined using multivariate regression model. The performance of the US Centers for Disease Control and Prevention (CDC) criteria (fever resolution, symptom improvement, and negative RADT) to predict loss of infectivity was also investigated.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In total, 121 participants (91 female [79.3%]; average age, 40 years) were enrolled. Most (n = 107, 88.4%) had received ≥3 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine doses, and 20 (16.5%) had COVID-19 previously. Viral culture positivity decreased from 71.9% (87/121) on day 5 of infection to 18.2% (22/121) on day 10. Participants with recurrent COVID-19 had a lower likelihood of infectivity than those with primary COVID-19 at each follow-up (day 5 odds ratio [OR], 0.14; P &lt; .001]; day 7 OR, 0.04; P = .003]) and were all non-infective by day 10 (P = .02). Independent predictors of infectivity included prior COVID-19 (adjusted OR [aOR] on day 5, 0.005; P = .003), an RT-PCR cycle threshold [Ct] value &lt;23 (aOR on day 5, 22.75; P &lt; .001) but not symptom improvement or RADT result.</jats:p>\n <jats:p>The CDC criteria would identify 36% (24/67) of all non-infectious individuals on day 7. However, 17% (5/29) of those meeting all the criteria had a positive viral culture.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Infectivity of recurrent COVID-19 is shorter than primary infections. Loss of infectivity algorithms could be optimized.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "McGill University Faculty of Medicine , Montréal , Canada"
}
],
"family": "Dzieciolowska",
"given": "Stefania",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Faculté de médecine, Université de Montréal , Montréal , Canada"
},
{
"name": "Laboratoire de Santé Publique du Québec , Sainte-Anne-de-Bellevue , Canada"
},
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Charest",
"given": "Hugues",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratoire de Santé Publique du Québec , Sainte-Anne-de-Bellevue , Canada"
},
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Roy",
"given": "Tonya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratoire de Santé Publique du Québec , Sainte-Anne-de-Bellevue , Canada"
},
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Fafard",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
},
{
"name": "Université Laval , Québec City , Canada"
}
],
"family": "Carazo",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratoire de Santé Publique du Québec , Sainte-Anne-de-Bellevue , Canada"
},
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Levade",
"given": "Ines",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "CHU de Québec—Université Laval , Québec City , Canada"
}
],
"family": "Longtin",
"given": "Jean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "McGill University Faculty of Medicine , Montréal , Canada"
},
{
"name": "Jewish General Hospital Sir Mortimer B. Davis , Montréal , Canada"
}
],
"family": "Parkes",
"given": "Leighanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratoire de Santé Publique du Québec , Sainte-Anne-de-Bellevue , Canada"
},
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Beaulac",
"given": "Sylvie Nancy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
}
],
"family": "Villeneuve",
"given": "Jasmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculté de médecine, Université de Montréal , Montréal , Canada"
},
{
"name": "Centre Hospitalier de l’Université de Montréal (CHUM) and CHUM Research Center , Montréal , Canada"
}
],
"family": "Savard",
"given": "Patrice",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Université Laval , Québec City , Canada"
}
],
"family": "Corbeil",
"given": "Jacques",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institut National de Santé Publique du Québec , Québec City , Canada"
},
{
"name": "Université Laval , Québec City , Canada"
}
],
"family": "De Serres",
"given": "Gaston",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4532-379X",
"affiliation": [
{
"name": "McGill University Faculty of Medicine , Montréal , Canada"
},
{
"name": "Jewish General Hospital Sir Mortimer B. Davis , Montréal , Canada"
},
{
"name": "Lady Davis Research Institute , Montréal , Canada"
}
],
"authenticated-orcid": false,
"family": "Longtin",
"given": "Yves",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T20:02:37Z",
"timestamp": 1693512157000
},
"deposited": {
"date-parts": [
[
2023,
9,
29
]
],
"date-time": "2023-09-29T13:34:26Z",
"timestamp": 1695994466000
},
"funder": [
{
"name": "Ministère de la Santé et des Services Sociaux (MSSS) du Québec"
},
{
"name": "Quebec’s Ministry of Health"
},
{
"DOI": "10.13039/100011094",
"doi-asserted-by": "publisher",
"name": "Public Health Agency of Canada"
},
{
"DOI": "10.13039/100011094",
"doi-asserted-by": "crossref",
"name": "(PHAC),"
},
{
"DOI": "10.13039/100013392",
"doi-asserted-by": "publisher",
"name": "MSSS"
},
{
"DOI": "10.13039/100011094",
"doi-asserted-by": "publisher",
"name": "PHAC"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
30
]
],
"date-time": "2023-09-30T09:49:53Z",
"timestamp": 1696067393004
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
9,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
7
]
],
"date-time": "2023-09-07T00:00:00Z",
"timestamp": 1694044800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciad535/51797932/ciad535.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciad535/51797932/ciad535.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
9,
7
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
7
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.3390/jcm9041225",
"article-title": "The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control",
"author": "Helmy",
"doi-asserted-by": "crossref",
"first-page": "1225",
"journal-title": "J Clin Med",
"key": "2023092912513658500_ciad535-B1",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1056/NEJMclde2100910",
"article-title": "Doctor, how long should I isolate?",
"author": "Kadire",
"doi-asserted-by": "crossref",
"first-page": "e47",
"journal-title": "N Engl J Med",
"key": "2023092912513658500_ciad535-B2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2008457",
"article-title": "Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility",
"author": "Arons",
"doi-asserted-by": "crossref",
"first-page": "2081",
"journal-title": "N Engl J Med",
"key": "2023092912513658500_ciad535-B3",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological assessment of hospitalized patients with COVID-2019",
"author": "Wolfel",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Nature",
"key": "2023092912513658500_ciad535-B4",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(20)30172-5",
"article-title": "SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis",
"author": "Cevik",
"doi-asserted-by": "crossref",
"first-page": "e13",
"journal-title": "Lancet Microbe",
"key": "2023092912513658500_ciad535-B5",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa638",
"article-title": "Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples",
"author": "Bullard",
"doi-asserted-by": "crossref",
"first-page": "2663",
"journal-title": "Clin Infect Dis",
"key": "2023092912513658500_ciad535-B6",
"volume": "71",
"year": "2020"
},
{
"author": "US Centers for Diseases Control and Prevention",
"key": "2023092912513658500_ciad535-B7"
},
{
"author": "European Center for Disease Control and Prevention",
"key": "2023092912513658500_ciad535-B8"
},
{
"DOI": "10.1056/NEJMc2202092",
"article-title": "Duration of shedding of culturable virus in SARS-CoV-2 Omicron (BA.1) infection",
"author": "Boucau",
"doi-asserted-by": "crossref",
"first-page": "275",
"journal-title": "N Engl J Med",
"key": "2023092912513658500_ciad535-B9",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30917-X",
"article-title": "COVID-19: the case for health-care worker screening to prevent hospital transmission",
"author": "Black",
"doi-asserted-by": "crossref",
"first-page": "1418",
"journal-title": "Lancet",
"key": "2023092912513658500_ciad535-B10",
"volume": "395",
"year": "2020"
},
{
"author": "World Health Organization",
"key": "2023092912513658500_ciad535-B11"
},
{
"DOI": "10.1016/j.jclinepi.2007.11.008",
"article-title": "The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies",
"author": "von Elm",
"doi-asserted-by": "crossref",
"first-page": "344",
"journal-title": "J Clin Epidemiol",
"key": "2023092912513658500_ciad535-B12",
"volume": "61",
"year": "2008"
},
{
"DOI": "10.1016/j.cmi.2021.02.014",
"article-title": "Prolonged SARS-CoV-2 cell culture replication in respiratory samples from patients with severe COVID-19",
"author": "Folgueira",
"doi-asserted-by": "crossref",
"first-page": "886",
"journal-title": "Clin Microbiol Infect",
"key": "2023092912513658500_ciad535-B13",
"volume": "27",
"year": "2021"
},
{
"article-title": "Infectivity of healthcare workers diagnosed with coronavirus disease 2019 (COVID-19) approximately 2 weeks after onset of symptoms: a cross-sectional study",
"author": "Longtin",
"first-page": "1",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "2023092912513658500_ciad535-B14",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1007/s10096-020-04106-0",
"article-title": "Culture of SARS-CoV-2 in a panel of laboratory cell lines, permissivity, and differences in growth profile",
"author": "Wurtz",
"doi-asserted-by": "crossref",
"first-page": "477",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "2023092912513658500_ciad535-B15",
"volume": "40",
"year": "2021"
},
{
"author": "CoV Sequencing Pipeline",
"key": "2023092912513658500_ciad535-B16"
},
{
"DOI": "10.1093/ve/veab064",
"article-title": "Assignment of epidemiological lineages in an emerging pandemic using the pangolin tool",
"author": "O'Toole",
"doi-asserted-by": "crossref",
"first-page": "veab064",
"journal-title": "Virus Evol",
"key": "2023092912513658500_ciad535-B17",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2022.10559",
"article-title": "Adequacy of serial self-performed SARS-CoV-2 rapid antigen detection testing for longitudinal mass screening in the workplace",
"author": "Papenburg",
"doi-asserted-by": "crossref",
"first-page": "e2210559",
"journal-title": "JAMA Netw Open",
"key": "2023092912513658500_ciad535-B18",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1038/s41591-022-01780-9",
"article-title": "Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults",
"author": "Killingley",
"doi-asserted-by": "crossref",
"first-page": "1031",
"journal-title": "Nat Med",
"key": "2023092912513658500_ciad535-B19",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1016/S0895-4356(00)00314-0",
"article-title": "Adjusting for multiple testing–when and how?",
"author": "Bender",
"doi-asserted-by": "crossref",
"first-page": "343",
"journal-title": "J Clin Epidemiol",
"key": "2023092912513658500_ciad535-B20",
"volume": "54",
"year": "2001"
},
{
"DOI": "10.1097/00001648-199001000-00010",
"article-title": "No adjustments are needed for multiple comparisons",
"author": "Rothman",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Epidemiology",
"key": "2023092912513658500_ciad535-B21",
"volume": "1",
"year": "1990"
},
{
"DOI": "10.1186/s12960-022-00764-7",
"article-title": "A global overview of healthcare workers’ turnover intention amid COVID-19 pandemic: a systematic review with future directions",
"author": "Poon",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "Hum Resour Health",
"key": "2023092912513658500_ciad535-B22",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2023.02.011",
"article-title": "Duration of viable virus shedding and polymerase chain reaction positivity of the SARS-CoV-2 Omicron variant in the upper respiratory tract: a systematic review and meta-analysis",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "228",
"journal-title": "Int J Infect Dis",
"key": "2023092912513658500_ciad535-B23",
"volume": "129",
"year": "2023"
},
{
"DOI": "10.1016/j.cmi.2022.07.009",
"article-title": "Duration of infectious shedding of SARS-CoV-2 Omicron variant and its relation with symptoms",
"author": "Keske",
"doi-asserted-by": "crossref",
"first-page": "221",
"journal-title": "Clin Microbiol Infect",
"key": "2023092912513658500_ciad535-B24",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7108a3",
"article-title": "Antigen test positivity after COVID-19 isolation—Yukon-Kuskokwim delta region, Alaska, January–February 2022",
"author": "Lefferts",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2023092912513658500_ciad535-B25",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2020.05.045",
"article-title": "Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: a retrospective cohort study",
"author": "Qi",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Int J Infect Dis",
"key": "2023092912513658500_ciad535-B26",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1101/2022.02.01.22269931",
"article-title": "High rates of rapid antigen test positivity after 5 days of isolation for COVID-19",
"author": "Landon",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv",
"key": "2023092912513658500_ciad535-B27",
"year": "2022"
},
{
"DOI": "10.1017/ice.2022.21",
"article-title": "Healthcare personnel frequently have positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen tests 5 days or more after diagnosis of coronavirus disease 2019 (COVID-19)",
"author": "Stiefel",
"doi-asserted-by": "crossref",
"first-page": "1985",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "2023092912513658500_ciad535-B28",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.3201/eid2805.220197",
"article-title": "Duration of infectious virus shedding by SARS-CoV-2 Omicron variant-infected vaccinees",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "998",
"journal-title": "Emerg Infect Dis",
"key": "2023092912513658500_ciad535-B29",
"volume": "28",
"year": "2022"
},
{
"author": "Kissler",
"key": "2023092912513658500_ciad535-B30"
},
{
"DOI": "10.1017/S0950268800048019",
"article-title": "The time course of the immune response to experimental coronavirus infection of man",
"author": "Callow",
"doi-asserted-by": "crossref",
"first-page": "435",
"journal-title": "Epidemiol Infect",
"key": "2023092912513658500_ciad535-B31",
"volume": "105",
"year": "1990"
},
{
"DOI": "10.1016/S1473-3099(22)00801-5",
"article-title": "Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression",
"author": "Bobrovitz",
"doi-asserted-by": "crossref",
"first-page": "556",
"journal-title": "Lancet Infect Dis",
"key": "2023092912513658500_ciad535-B32",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciaa1616",
"article-title": "Repeated coronavirus disease 2019 molecular testing: correlation of severe acute respiratory syndrome coronavirus 2 culture with molecular assays and cycle thresholds",
"author": "Gniazdowski",
"doi-asserted-by": "crossref",
"first-page": "e860",
"journal-title": "Clin Infect Dis",
"key": "2023092912513658500_ciad535-B33",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2027040",
"article-title": "Duration of culturable SARS-CoV-2 in hospitalized patients with Covid-19",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "N Engl J Med",
"key": "2023092912513658500_ciad535-B34",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1017/ice.2021.502",
"article-title": "Persistence of infectivity in elderly individuals diagnosed with severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection 10 days after onset of symptoms: a cross-sectional study",
"author": "Longtin",
"doi-asserted-by": "crossref",
"first-page": "659",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "2023092912513658500_ciad535-B35",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27206",
"article-title": "Cycle threshold values in RT-PCR to determine dynamics of SARS-CoV-2 viral load: an approach to reduce the isolation period for COVID-19 patients",
"author": "Aranha",
"doi-asserted-by": "crossref",
"first-page": "6794",
"journal-title": "J Med Virol",
"key": "2023092912513658500_ciad535-B36",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1764",
"article-title": "Viral cultures for COVID-19 infectious potential assessment—a systematic review",
"author": "Jefferson",
"doi-asserted-by": "crossref",
"first-page": "e3884",
"journal-title": "Clin Infect Dis",
"key": "2023092912513658500_ciad535-B37",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciac510",
"article-title": "Viral dynamics of omicron and delta severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with implications for timing of release from isolation: a longitudinal cohort study",
"author": "Bouton",
"doi-asserted-by": "crossref",
"first-page": "e227",
"journal-title": "Clin Infect Dis",
"key": "2023092912513658500_ciad535-B38",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1128/JCM.00469-21",
"article-title": "Can testing predict SARS-CoV-2 infectivity? The potential for certain methods to be surrogates for replication-competent virus",
"author": "Binnicker",
"doi-asserted-by": "crossref",
"first-page": "e0046921",
"journal-title": "J Clin Microbiol",
"key": "2023092912513658500_ciad535-B39",
"volume": "59",
"year": "2021"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciad535/7262516"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Timing and Predictors of Loss of Infectivity Among Healthcare Workers With Mild Primary and Recurrent Coronavirus Disease 2019 (COVID-19): A Prospective Observational Cohort Study",
"type": "journal-article"
}