Sep 14 2022 |
et al., Infection, doi:10.1007/s15010-022-01904-w | DFV890: a new oral NLRP3 inhibitor—tested in an early phase 2a randomised clinical trial in patients with COVID-19 pneumonia and impaired respiratory function |
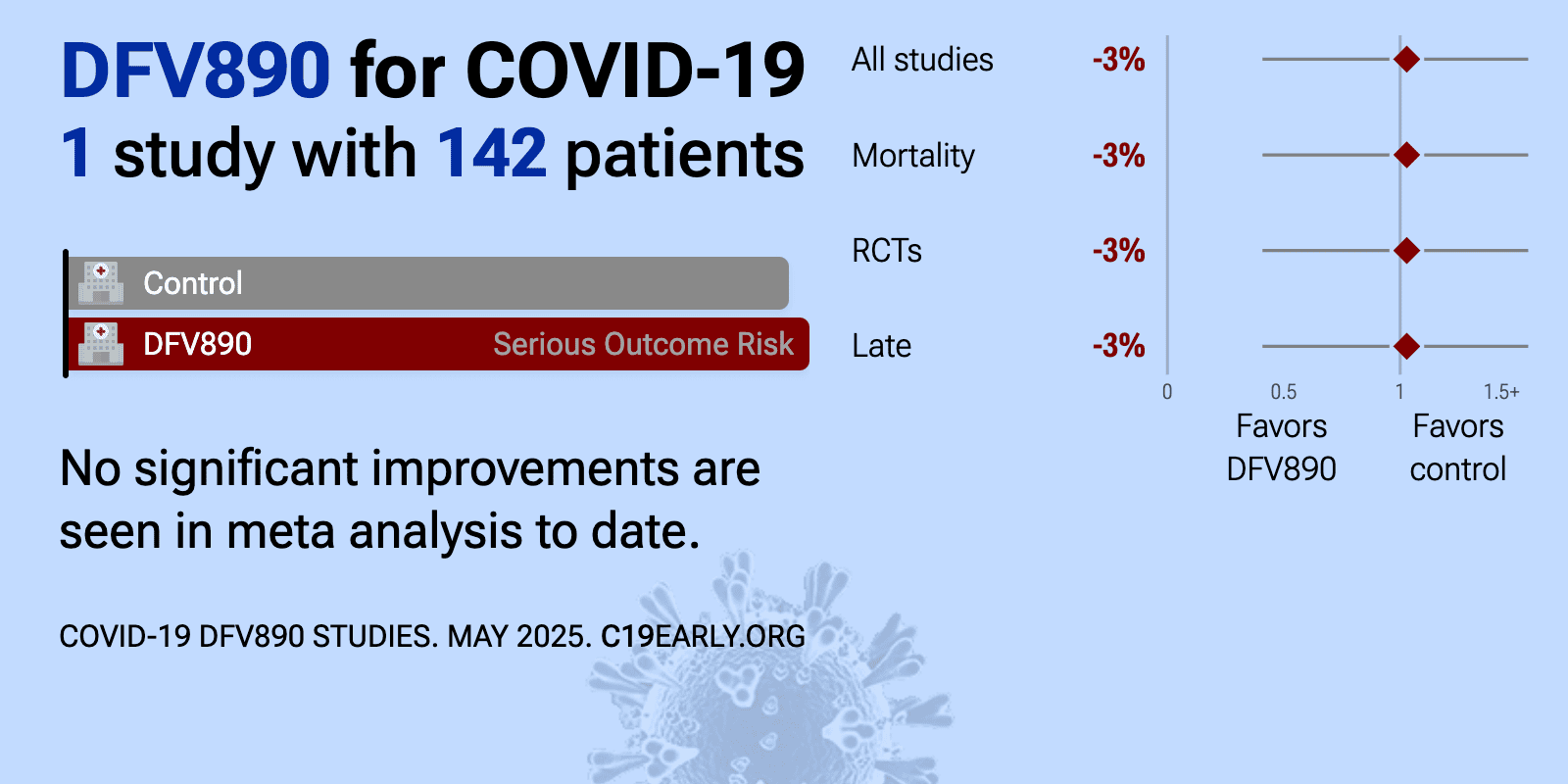
| 3% higher mortality (p=1) and 1% worse recovery (p=0.95). RCT 143 hospitalized patients with COVID-19 pneumonia showing no significant difference in APACHE II scores with DFV890 (NLRP3 inhibitor). | ||