Proton pump inhibitors associated with severe COVID‐19 among two‐dose but not three‐dose vaccine recipients
et al., Journal of Gastroenterology and Hepatology, doi:10.1111/jgh.16601, May 2024
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
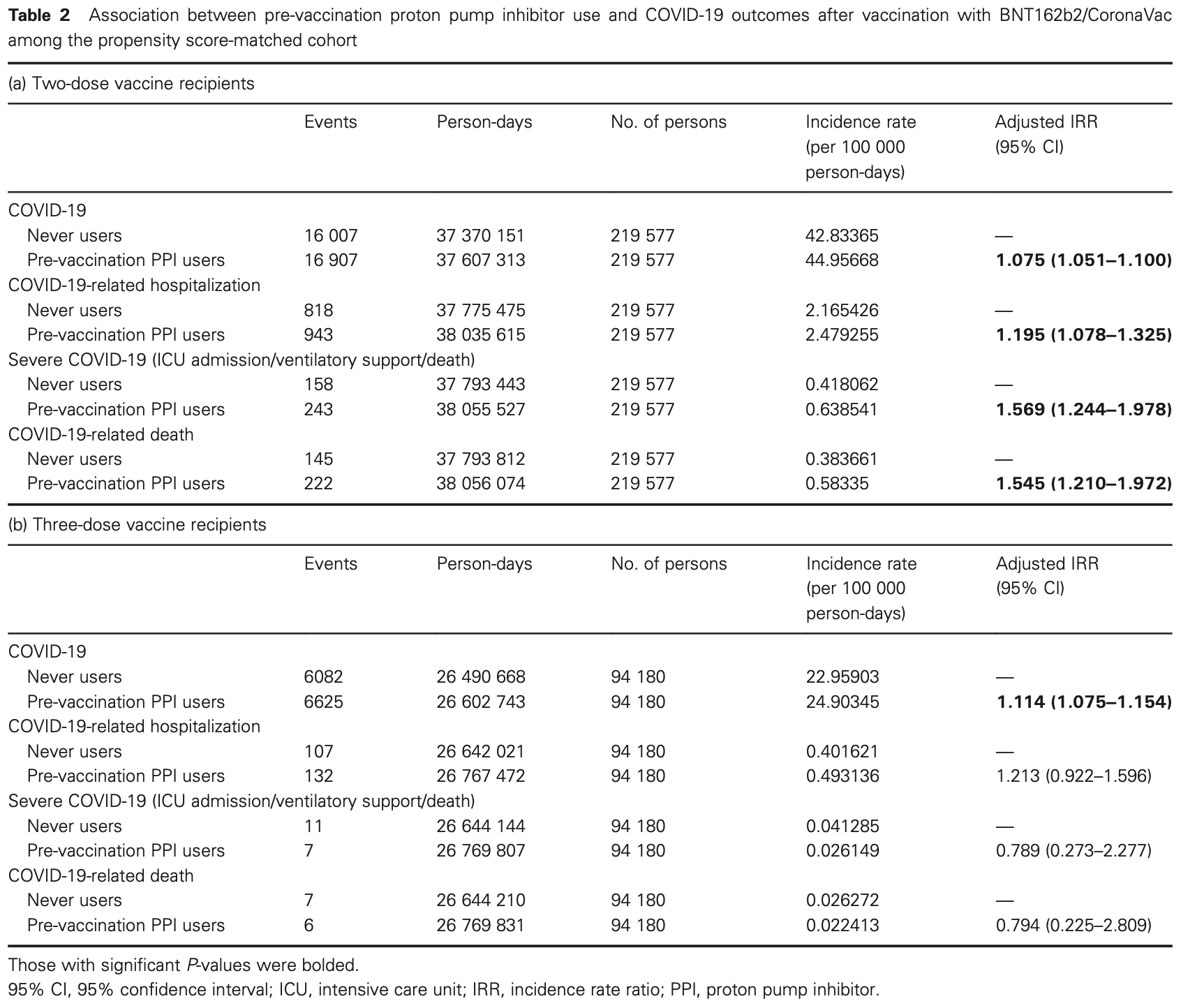
Retrospective 627,514 patients in Hong Kong showing slightly higher risk of COVID-19 with pre-vaccination proton pump inhibitor (PPI) use in two-dose or three-dose vaccine recipients, and higher risk of hospitalization and severe outcomes only in two-dose recipients.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of death, 49.5% higher, RR 1.49, p = 0.005, adjusted per study, all patients.
|
|
risk of death, 54.5% higher, RR 1.54, p < 0.001, adjusted per study, 2 dose.
|
|
risk of death, 20.6% lower, RR 0.79, p = 0.73, treatment 6 of 94,180 (0.0%), control 7 of 95,180 (0.0%), NNT 101656, adjusted per study, 3 dose.
|
|
risk of severe case, 36.3% higher, RR 1.36, p = 0.27, adjusted per study, all patients.
|
|
risk of severe case, 56.9% higher, RR 1.57, p < 0.001, adjusted per study, 2 dose.
|
|
risk of severe case, 21.1% lower, RR 0.79, p = 0.67, treatment 7 of 94,180 (0.0%), control 11 of 95,180 (0.0%), adjusted per study, 3 dose.
|
|
risk of hospitalization, 19.7% higher, RR 1.20, p < 0.001, adjusted per study, all patients.
|
|
risk of hospitalization, 19.5% higher, RR 1.20, p < 0.001, adjusted per study, 2 dose.
|
|
risk of hospitalization, 21.3% higher, RR 1.21, p = 0.17, treatment 132 of 94,180 (0.1%), control 107 of 95,180 (0.1%), adjusted per study, 3 dose.
|
|
risk of case, 9.1% higher, RR 1.09, p < 0.001, adjusted per study, all patients.
|
|
risk of case, 7.5% higher, RR 1.07, p < 0.001, adjusted per study, 2 dose.
|
|
risk of case, 11.4% higher, RR 1.11, p < 0.001, treatment 6,625 of 94,180 (7.0%), control 6,082 of 95,180 (6.4%), adjusted per study, 3 dose.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cheung et al., 5 May 2024, retrospective, China, peer-reviewed, mean age 65.6, 6 authors, study period 23 February, 2021 - 31 March, 2022.
Contact: waikleung@hku.hk, ewchan@hku.hk.
Proton pump inhibitors associated with severe COVID‐19 among two‐dose but not three‐dose vaccine recipients
Journal of Gastroenterology and Hepatology, doi:10.1111/jgh.16601
Background and Aim: Proton pump inhibitors (PPIs) may increase the risk of COVID-19 among non-vaccinated subjects via various mechanisms, including gut dysbiosis. We aimed to investigate whether PPIs also affect the clinical outcomes of COVID-19 among vaccine recipients. Methods: This was a territory-wide cohort study of 3 272 286 vaccine recipients (aged ≥ 18 years) of ≥ 2 doses of either BNT162b2 or CoronaVac. Exclusion criteria included prior gastrointestinal surgery, immunocompromised status, and prior COVID-19. The primary outcome was COVID-19, and secondary outcomes included COVID-19-related hospitalization and severe infection (composite of intensive care unit admission, ventilatory support, and/or death). Covariates include age, sex, the Charlson Comorbidity Index, comorbidities, and concomitant medication use. Subjects were followed from index date (first dose of vaccination) until outcome occurrence, death, additional dose of vaccination, or March 31, 2022. Exposure was pre-vaccination PPI use (any prescription within 90 days before the index date). Propensity score (PS) matching and a Poisson regression model were used to estimate the adjusted incidence rate ratio (aIRR) of outcomes with PPI use. Results: Among 439 154 PS-matched two-dose vaccine recipients (mean age: 65.3 years; male: 45.7%) with a median follow-up of 6.8 months (interquartile range: 2.6-7.9), PPI exposure was associated with a higher risk of COVID-19 (aIRR: 1.08; 95% confidence interval [95% CI]: 1.05-1.10), hospitalization (aIRR: 1.20; 95% CI: 1.08-1.33), and severe infection (aIRR: 1.57; 95% CI: 1.24-1.98). Among 188 360 PS-matched three-dose vaccine recipients (mean age: 62.5 years; male: 49.0%; median follow-up: 9.1 months [interquartile range: 8.0-10.9]), PPIs were associated with higher infection risk (aIRR: 1.11; 95% CI: 1.08-1.15) but not other outcomes. Conclusions: Although PPI use was associated with a higher COVID-19 risk, severe infection was limited to two-dose but not three-dose vaccine recipients.
Supporting information Additional supporting information may be found online in the Supporting Information section at the end of the article. S1 . ICD-9 coding for covariates. Table S2 . Baseline characteristics between proton pump inhibitor users and non-users before propensity score matching. Table S3 . Sensitivity analysis based on modified index date: baseline characteristics between proton pump inhibitor users and non-users after propensity score matching. Table S4 . Sensitivity analysis based on modified index date: association between pre-vaccination proton pump inhibitor use and COVID-19 outcomes after vaccination with BNT162b2/CoronaVac among the propensity-score matched cohort.
References
Adachi, Katsube, Kawamura, CYP2C19 genotype status and intragastric pH during dosing with lansoprazole or rabeprazole, Aliment. Pharmacol. Ther
Almario, Chey, Spiegel, Increased risk of COVID-19 among users of proton pump inhibitors, Am. J. Gastroenterol
Asamoah-Boaheng, Goldfarb, Karim, The relationship between anti-spike SARS-CoV-2 antibody levels and risk of breakthrough COVID-19 among fully vaccinated adults, J Infect Dis
Barda, Dagan, Cohen, Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study, Lancet
Bretthauer, Løberg, Wieszczy, Effect of colonoscopy screening on risks of colorectal cancer and related death, N. Engl. J. Med
Cheung, Chan, Seto, Wong, Leung, ACE (angiotensin-converting enzyme) inhibitors/angiotensin receptor blockers are associated with lower colorectal cancer risk: a territory-wide study with propensity score analysis, Hypertension
Cheung, Chen, Chan, Seto, Wong et al., Nonsteroidal anti-inflammatory drugs but not aspirin are associated with a lower risk of post-colonoscopy colorectal cancer, Aliment. Pharmacol. Ther
Cheung, Chen, Seto, Leung, Epidemiology, characteristics, and survival of post-colonoscopy colorectal cancer in Asia: a population-based study, J. Gastroenterol. Hepatol
Cheung, Hung, Leung, Association between angiotensin blockade and COVID-19 severity in Hong Kong, Cmaj
Cheung, Hung, Leung, Association between famotidine use and COVID-19 severity in Hong Kong: a territory-wide study, Gastroenterology
Cheung, Lam, Hui, Effect of moderate-to-severe hepatic steatosis on neutralising antibody response among BNT162b2 and CoronaVac recipients, Clin. Mol. Hepatol
Cheung, Lam, Seto, Leung, Use of antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in hepatocellular carcinoma, Liver Cancer
Cheung, Lam, Zhang, Association between recent usage of antibiotics and immunogenicity within six months after COVID-19 vaccination, Vaccines
Cheung, Leung, Seto, Application of Big Data analysis in gastrointestinal research, World J. Gastroenterol
Cheung, Mok, Mao, COVID-19 vaccine immunogenicity among chronic liver disease patients and liver transplant recipients: a meta-analysis, Clin. Mol. Hepatol
Cheung, Yan, Lam, Antibiotic use prior to COVID-19 vaccine is associated with higher risk of COVID-19 and adverse outcomes: a propensity-scored matched territory-wide cohort, Vaccines
Chua, Kwan, Chui, Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination, Clin. Infect. Dis
Dhar, Mohanty, Gut microbiota and Covid-19-possible link and implications, Virus Res
Freedberg, Conigliaro, Wang, Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study, Gastroenterology
Haas, Angulo, Mclaughlin, Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data, Lancet
Hagan, Cortese, Rouphael, Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans, Cell
Imhann, Bonder, Vila, Proton pump inhibitors affect the gut microbiome, Gut
Jara, Undurraga, González, Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile, N. Engl. J. Med
Laheij, Sturkenboom, Hassing, Dieleman, Stricker et al., Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs, JAMA
Lai, Huang, Chui, Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong, Nat. Commun
Lee, Ha, Yeniova, Severe clinical outcomes of COVID-19 associated with proton pump inhibitors: a nationwide cohort study with propensity score matching, Gut
Li, Tong, Wong, Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study, Gut
Lim, Mak, Leung, Cowling, Peiris, Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19, Lancet Microbe
Lin, Jiang, Zhang, Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection, Gut
Lynn, Benson, Lynn, Pulendran, Modulation of immune responses to vaccination by the microbiota: implications and potential mechanisms, Nat. Rev. Immunol
Munro, Janani, Cornelius, Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial, Lancet
Nemet, Kliker, Lustig, Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection, N. Engl. J. Med
Nordström, Ballin, Nordström, Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: a retrospective, total population cohort study in Sweden, Lancet
Oh, Ravindran, Chassaing, TLR5-mediated sensing of gut microbiota is necessary for antibody responses to seasonal influenza vaccination, Immunity
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, N. Engl. J. Med
Pranata, Huang, Lawrensia, Proton pump inhibitor on susceptibility to COVID-19 and its severity: a systematic review and meta-analysis, Pharmacol. Rep
Schaupp, Muth, Rogell, Microbiota-induced type I interferons instruct a poised basal state of dendritic cells, Cell
Trifan, Stanciu, Girleanu, Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and metaanalysis, World J. Gastroenterol
Wan, Chui, Lai, Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study, Lancet Infect. Dis
Zhou, Li, Zhao, Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus, Sci. Adv
DOI record:
{
"DOI": "10.1111/jgh.16601",
"ISSN": [
"0815-9319",
"1440-1746"
],
"URL": "http://dx.doi.org/10.1111/jgh.16601",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background and Aim</jats:title><jats:p>Proton pump inhibitors (PPIs) may increase the risk of COVID‐19 among non‐vaccinated subjects via various mechanisms, including gut dysbiosis. We aimed to investigate whether PPIs also affect the clinical outcomes of COVID‐19 among vaccine recipients.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>This was a territory‐wide cohort study of 3 272 286 vaccine recipients (aged ≥ 18 years) of ≥ 2 doses of either BNT162b2 or CoronaVac. Exclusion criteria included prior gastrointestinal surgery, immunocompromised status, and prior COVID‐19. The primary outcome was COVID‐19, and secondary outcomes included COVID‐19‐related hospitalization and severe infection (composite of intensive care unit admission, ventilatory support, and/or death). Covariates include age, sex, the Charlson Comorbidity Index, comorbidities, and concomitant medication use. Subjects were followed from index date (first dose of vaccination) until outcome occurrence, death, additional dose of vaccination, or March 31, 2022. Exposure was pre‐vaccination PPI use (any prescription within 90 days before the index date). Propensity score (PS) matching and a Poisson regression model were used to estimate the adjusted incidence rate ratio (aIRR) of outcomes with PPI use.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Among 439 154 PS‐matched two‐dose vaccine recipients (mean age: 65.3 years; male: 45.7%) with a median follow‐up of 6.8 months (interquartile range: 2.6–7.9), PPI exposure was associated with a higher risk of COVID‐19 (aIRR: 1.08; 95% confidence interval [95% CI]: 1.05–1.10), hospitalization (aIRR: 1.20; 95% CI: 1.08–1.33), and severe infection (aIRR: 1.57; 95% CI: 1.24–1.98). Among 188 360 PS‐matched three‐dose vaccine recipients (mean age: 62.5 years; male: 49.0%; median follow‐up: 9.1 months [interquartile range: 8.0–10.9]), PPIs were associated with higher infection risk (aIRR: 1.11; 95% CI: 1.08–1.15) but not other outcomes.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Although PPI use was associated with a higher COVID‐19 risk, severe infection was limited to two‐dose but not three‐dose vaccine recipients.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/jgh.16601"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-12-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-04-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-05-05"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4838-378X",
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine The University of Hong Kong, Queen Mary Hospital Hong Kong"
}
],
"authenticated-orcid": false,
"family": "Cheung",
"given": "Ka Shing",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine The University of Hong Kong Hong Kong"
}
],
"family": "Yan",
"given": "Vincent K C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine The University of Hong Kong Hong Kong"
}
],
"family": "Ye",
"given": "Xuxiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine The University of Hong Kong, Queen Mary Hospital Hong Kong"
}
],
"family": "Hung",
"given": "Ivan F N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine The University of Hong Kong Hong Kong"
},
{
"name": "Laboratory of Data Discovery for Health (D<sup>2</sup>4H), Hong Kong Science and Technology Park Hong Kong"
},
{
"name": "Department of Pharmacy The University of Hong Kong‐Shenzhen Hospital Shenzhen China"
},
{
"name": "The University of Hong Kong Shenzhen Institute of Research and Innovation Shenzhen China"
}
],
"family": "Chan",
"given": "Esther W",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5993-1059",
"affiliation": [
{
"name": "Department of Medicine, School of Clinical Medicine The University of Hong Kong, Queen Mary Hospital Hong Kong"
}
],
"authenticated-orcid": false,
"family": "Leung",
"given": "Wai K",
"sequence": "additional"
}
],
"container-title": "Journal of Gastroenterology and Hepatology",
"container-title-short": "J of Gastro and Hepatol",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
5,
6
]
],
"date-time": "2024-05-06T02:34:26Z",
"timestamp": 1714962866000
},
"deposited": {
"date-parts": [
[
2024,
5,
6
]
],
"date-time": "2024-05-06T02:34:30Z",
"timestamp": 1714962870000
},
"funder": [
{
"DOI": "10.13039/501100005407",
"award": [
"COVID1903011"
],
"doi-asserted-by": "publisher",
"name": "Food and Health Bureau"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
11
]
],
"date-time": "2024-05-11T03:09:38Z",
"timestamp": 1715396978474
},
"is-referenced-by-count": 1,
"issued": {
"date-parts": [
[
2024,
5,
5
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
5,
5
]
],
"date-time": "2024-05-05T00:00:00Z",
"timestamp": 1714867200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jgh.16601",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2024,
5,
5
]
]
},
"published-online": {
"date-parts": [
[
2024,
5,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/S0140-6736(21)00947-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_2_1"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_3_1"
},
{
"DOI": "10.1056/NEJMoa2107715",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_4_1"
},
{
"DOI": "10.1038/s41577-021-00554-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_5_1"
},
{
"DOI": "10.1016/j.cell.2019.08.010",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_6_1"
},
{
"article-title": "Association between recent usage of antibiotics and immunogenicity within six months after COVID‐19 vaccination",
"author": "Cheung KS",
"first-page": "10",
"journal-title": "Vaccines (Basel)",
"key": "e_1_2_7_7_1",
"year": "2022"
},
{
"DOI": "10.3390/vaccines11081341",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_8_1"
},
{
"DOI": "10.14309/ajg.0000000000000798",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_9_1"
},
{
"DOI": "10.1136/gutjnl-2020-322248",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_10_1"
},
{
"DOI": "10.1056/NEJMoa2208375",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_11_1"
},
{
"DOI": "10.3748/wjg.v23.i35.6500",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_12_1"
},
{
"DOI": "10.1001/jama.292.16.1955",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_13_1"
},
{
"DOI": "10.1016/S1473-3099(21)00451-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_14_1"
},
{
"DOI": "10.1093/cid/ciab989",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_15_1"
},
{
"DOI": "10.1038/s41467-022-28068-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_16_1"
},
{
"DOI": "10.1136/gutjnl-2021-326860",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_17_1"
},
{
"DOI": "10.1161/HYPERTENSIONAHA.120.15317",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_18_1"
},
{
"DOI": "10.1111/jgh.14674",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_19_1"
},
{
"DOI": "10.1111/apt.15693",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_20_1"
},
{
"DOI": "10.1159/000518090",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_21_1"
},
{
"DOI": "10.3350/cmh.2022.0087",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_22_1"
},
{
"DOI": "10.3350/cmh.2022.0082",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_23_1"
},
{
"DOI": "10.1053/j.gastro.2020.05.053",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_24_1"
},
{
"DOI": "10.1053/j.gastro.2020.05.098",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_25_1"
},
{
"DOI": "10.1503/cmaj.75865",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_26_1"
},
{
"DOI": "10.3748/wjg.v25.i24.2990",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_27_1"
},
{
"DOI": "10.1136/gutjnl-2020-321013",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_28_1"
},
{
"DOI": "10.1016/j.virusres.2020.198018",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_29_1"
},
{
"DOI": "10.1126/sciadv.aao4966",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_30_1"
},
{
"DOI": "10.1136/gutjnl-2015-310376",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_31_1"
},
{
"DOI": "10.1016/j.immuni.2014.08.009",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_32_1"
},
{
"DOI": "10.1016/j.cell.2020.04.022",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_33_1"
},
{
"DOI": "10.1016/S0140-6736(21)02717-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_34_1"
},
{
"DOI": "10.1056/NEJMc2119358",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_35_1"
},
{
"DOI": "10.1016/S0140-6736(21)02249-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_36_1"
},
{
"article-title": "The relationship between anti‐spike SARS‐CoV‐2 antibody levels and risk of breakthrough COVID‐19 among fully vaccinated adults",
"author": "Asamoah‐Boaheng M",
"journal-title": "J Infect Dis",
"key": "e_1_2_7_37_1",
"year": "2022"
},
{
"DOI": "10.1016/S2666-5247(21)00177-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_38_1"
},
{
"article-title": "Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID‐19 vaccine: a retrospective, total population cohort study in Sweden",
"author": "Nordström P",
"journal-title": "Lancet",
"key": "e_1_2_7_39_1",
"year": "2022"
},
{
"DOI": "10.1007/s43440-021-00263-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_40_1"
},
{
"DOI": "10.1046/j.1365-2036.2000.00840.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_7_41_1"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jgh.16601"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Proton pump inhibitors associated with severe COVID‐19 among two‐dose but not three‐dose vaccine recipients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}