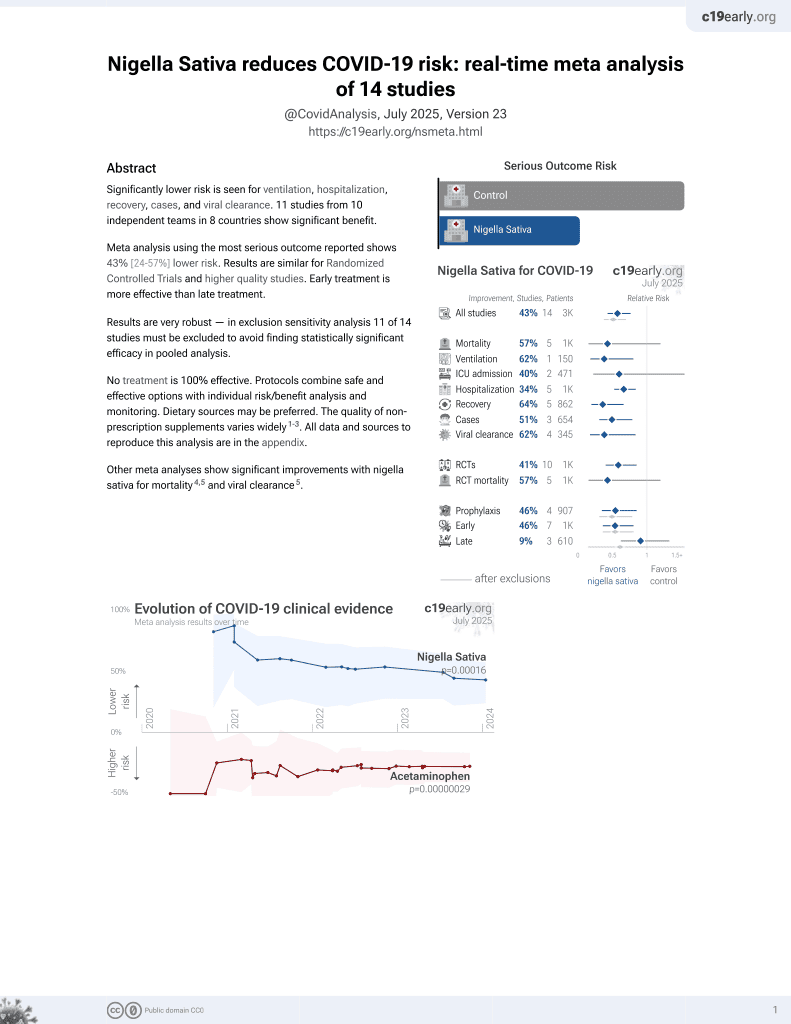
Efficacy of polyherbal formulations for prevention of COVID-19 infection in high-risk subjects: A randomized open-label controlled clinical trial
et al., Phytotherapy Research, doi:10.1002/ptr.7531, CTRI/2020/08/027222, Jul 2022
14th treatment shown to reduce risk in
January 2021, now with p = 0.00016 from 14 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 251 high-risk individuals in India, mostly with direct contact with COVID-19 positive patients, testing polyherbal formulations Infuza, which includes nigella sativa, and Kulzam. Both formulations showed lower risk, without statisical significance, while the best results were from the combination of both.
|
risk of case, 49.0% lower, RR 0.51, p = 0.36, treatment 4 of 52 (7.7%), control 8 of 53 (15.1%), NNT 14, Infuza.
|
|
risk of case, 87.0% lower, RR 0.13, p = 0.03, treatment 1 of 51 (2.0%), control 8 of 53 (15.1%), NNT 7.6, Infuza and Kulzam.
|
|
risk of case, 74.0% lower, RR 0.26, p = 0.09, treatment 2 of 51 (3.9%), control 8 of 53 (15.1%), NNT 9.0, Kulzam.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chandra et al., 5 Jul 2022, Randomized Controlled Trial, India, peer-reviewed, 12 authors, study period 18 September, 2020 - 21 May, 2021, this trial uses multiple treatments in the treatment arm (combined with Infuza polyherbal formulation) - results of individual treatments may vary, trial CTRI/2020/08/027222.
Contact: mridududeja@yahoo.com.
Efficacy of polyherbal formulations for prevention of COVID ‐19 infection in high‐risk subjects: A randomized open‐label controlled clinical trial
Phytotherapy Research, doi:10.1002/ptr.7531
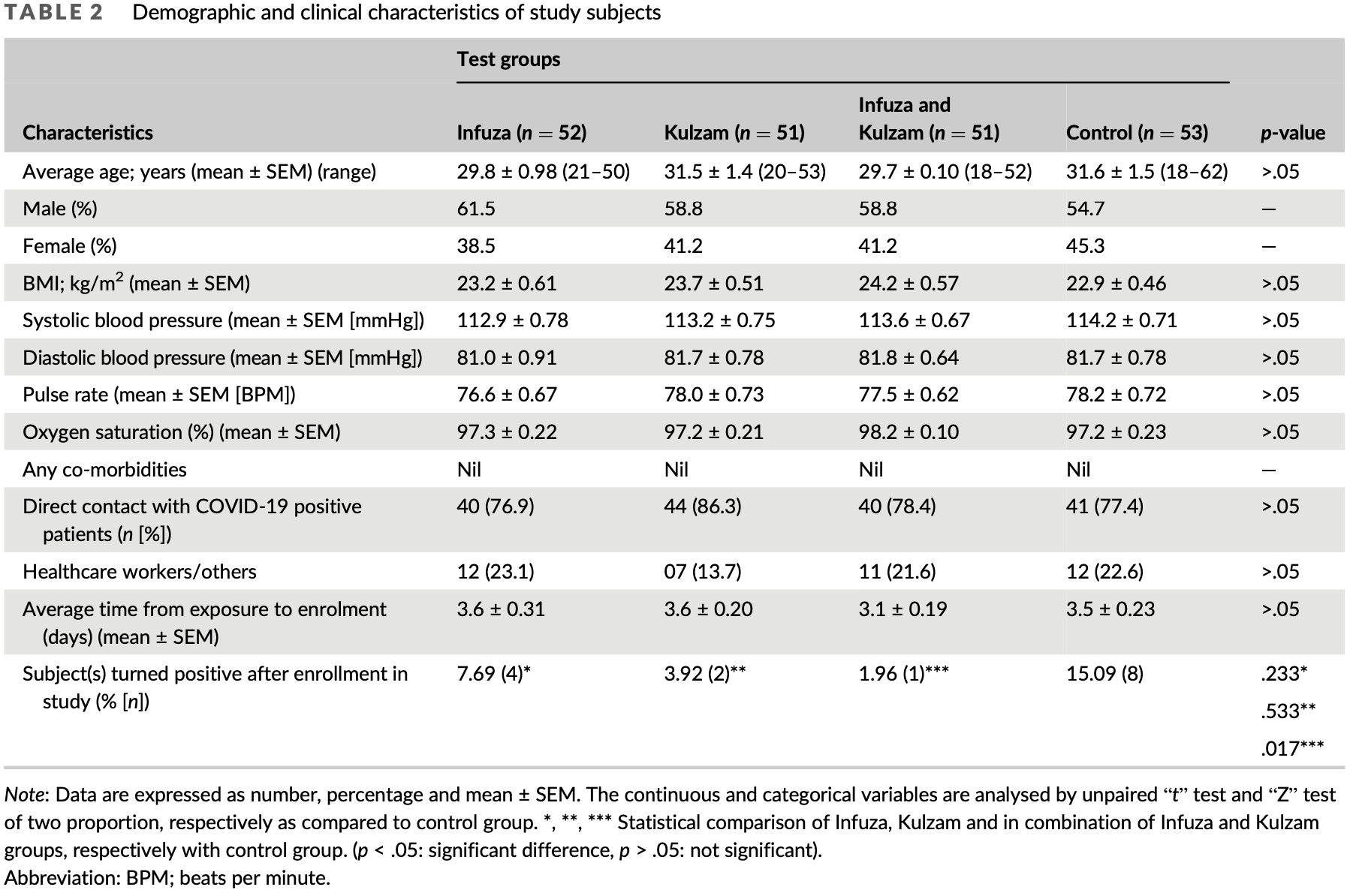
COVID-19 is arguably the biggest health crisis the world has faced in the 21st century. Therefore, two of the polyherbal formulations, Infuza and Kulzam were assessed for the prevention of COVID-19 infection as a repurposed medication. Four hundred seven high-risk subjects were recruited in the present open-label randomized controlled clinical trial for eligibility. After assessment for eligibility, remaining 251 subjects were randomized to the test and control groups. Further, 52 high-risk subjects in Infuza, 51 in Kulzam, 51 in Infuza & Kulzam and 53 in control group completed the 14 days of intervention/assessment. The phenotyping of lymphocytes at baseline (0 day) and after 14 days of treatment was carried out by flow cytometry assays. A total of 15.09% high-risk subjects in control group turned positive as compared to only 7.69% in Infuza, 3.92% in Kulzam and 1.96% in Infuza & Kulzam groups. The rate of conversion to COVID-19 infection in Infuza & Kulzam group was minimal and statistically significant as compared to control group (p0.017). No significant changes in phenotype of lymphocytes (T, B, NK cells), absolute lymphocyte count and cytokine levels were found in study groups. However, there was a decreasing trend of hs-CRP level in high-risk subjects after intervention of polyherbal formulations for 14 days. The combination of Infuza and Kulzam may synergistically prevent COVID-19 infection in high-risk subjects of COVID-19.
AUTHOR CONTRIBUTIONS Conceptualization: Mridu Dudeja, Kailash Chandra, Ayan Kumar Das, Naushad Ali Rana, Santosh Joshi, Asad Mueed; Data curation: Sumeera Banday, Mohini Arora, Shashank Agarwal, Santosh Joshi, Kailash Chandra; Formal analysis: Kailash Chandra, Varun Kashyap, Ayan Kumar Das, Sumeera Banday; Investigation: Mohini Arora, Santosh Joshi, Kailash Chandra, Ayan Kumar Das; Methodology: Ayan Kumar Das, Kailash Chandra, Varun Kashyap, Sonal Jain, Shashank
CONFLICT OF INTEREST We confirm that there is no conflict of interest associated with this publication. The medications were provided by M/S Hamdard Laboratories (Medicine Division), India. Clinical trial was conducted at Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital, New Delhi. M/S Hamdard Laboratories is not involved in any aspect of clinical trial reported in the study.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article. How to cite this article: Chandra, K., Das, A. K., Banday, S., Rana, N. A., Arora, M., Jain, S., Islam, F., Agarwal, S., Kashyap, V., Joshi, S., Mueed, A., & Dudeja, M. (2022) .
References
Alabboud, Javadmanesh, In silico study of various antiviral drugs, vitamins, and natural substances as potential binding compounds with SARS-CoV-2 main protease, DYSONA -Life Science, doi:10.30493/DLS.2020.225404
Andrade, De Rangel, Santos, Freitas, De Soares et al., Repurposing approved drugs for guiding COVID-19 prophylaxis: A systematic review, Frontiers in Pharmacology, doi:10.3389/fphar.2020.590598
Basu, Sarkar, Maulik, Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2, Scientific Reports, doi:10.1038/s41598-020-74715-4
Calder, Carr, Gombart, Eggersdorfer, Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections, Nutrients, doi:10.3390/nu12041181
Chandra, Jain, Azhar, Khan, Alam et al., Effect of augmented glycation in mobilization of plasma free fatty acids in type 2 diabetes mellitus, Diabetes and Metabolic Syndrome, doi:10.1016/j.dsx.2020.07.028
Chandra, Khan, Jetley, Ahmad, Jain, Antidiabetic, toxicological, and metabolomic profiling of aqueous extract of Cichorium intybus seeds, Pharmacognosy Magazine, doi:10.4103/pm.pm_583_17
Cohen, Hydroxychloroquine for the prevention of Covid-19 -Searching for evidence, New England Journal of Medicine, doi:10.1056/nejme2020388
Fiore, Eisenhut, Krausse, Ragazzi, Pellati et al., Antiviral effects of Glycyrrhiza species, Phytotherapy Research, doi:10.1002/PTR.2295
Forman, Shah, Jeurissen, Jit, Mossialos, COVID-19 vaccine challenges: What have we learned so far and what remains to be done?, Health Policy, doi:10.1016/j.healthpol.2021.03.013
Han, Xu, Cheng, Zhong, Yuan et al., Descriptive, retrospective study of the clinical characteristics of asymptomatic COVID-19 patients, MSphere, doi:10.1128/msphere.00922-20
Hasan, Ara, Mondal, Kabir, Phytochemistry, pharmacological activity, and potential health benefits of Glycyrrhiza glabra, Heliyon, doi:10.1016/J.HELIYON.2021.E07240
Jorge, Hydroxychloroquine in the prevention of COVID-19 mortality, The Lancet Rheumatology, doi:10.1016/S2665-9913(20)30390-8
Khanna, Kohli, Kaur, Bhardwaj, Bhardwaj et al., Herbal immune-boosters: Substantial warriors of pandemic Covid-19 battle, Phytomedicine, doi:10.1016/j.phymed.2020.153361
Koshak, Koshak, Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: A mini review of in silico studies, Current Therapeutic Research, doi:10.1016/j.curtheres.2020.100602
Ma, Chen, Guo, Liu, Chen et al., Prevention and treatment of infectious diseases by traditional Chinese medicine: A commentary, APMIS, doi:10.1111/apm.12928
Maurya, Sharma, Evaluation of traditional ayurvedic Kadha for prevention and management of the novel coronavirus (SARS-CoV-2) using in silico approach, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2020.1852119
Mohammadi, Shaghaghi, Inhibitory effect of eight secondary metabolites from conventional medicinal plants on COVID-19 virus protease by molecular docking analysis, ChemRxiv, doi:10.26434/CHEMRXIV.11987475.V1
Mukherjee, Mao, Mohfw, Ramos ; Tovar, Muriel, Compendium of antiviral medicinal plants of north East India, doi:10.1016/B978-0-12-814466-4.00009-4
Najmul, Rampuri, None, Khazain ul Advia
References Akram, Tahir, Shah, Mahmood, Altaf et al., Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: A systematic review, Phytotherapy Research, doi:10.1002/PTR.6024
Richman, COVID-19 vaccines: Implementation, limitations and opportunities, Global Health & Medicine, doi:10.35772/ghm.2021.01010
Saboo, Immunomodulator in traditional healthcare system, doi:10.5772/INTECHOPEN.94965
Salvi, Patankar, Emerging pharmacotherapies for COVID-19, Biomedicine & Pharmacotherapy, doi:10.1016/J.BIOPHA.2020.110267
Shah, Rasul, Yousaf, Haris, Faheem et al., Combination of natural antivirals and potent immune invigorators: A natural remedy to combat COVID-19, Phytotherapy Research, doi:10.1002/PTR.7228
Sharanya, Sabu, Haridas, Potent phytochemicals against COVID-19 infection from phyto-materials used as antivirals in complementary medicines: A review, Future Journal of Pharmaceutical Sciences, doi:10.1186/S43094-021-00259-7
Sharma, Kaur, Bioactive molecules from eucalyptus essential oil as potential inhibitors of COVID-19 corona virus infection by molecular docking studies, Kragujevac Journal of Science
Wang, C-reactive protein levels in the early stage of COVID-19, Médecine et Maladies Infectieuses, doi:10.1016/j.medmal.2020.03.007
Yeh, Chih Wang, Chai Chiang, Shieh, Hong Yen et al., Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines, Journal of Ethnopharmacology, doi:10.1016/j.jep.2013.04.040
DOI record:
{
"DOI": "10.1002/ptr.7531",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7531",
"alternative-id": [
"10.1002/ptr.7531"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-06-05"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-07-05"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Biochemistry Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Chandra",
"given": "Kailash",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Microbiology Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Das",
"given": "Ayan Kumar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Banday",
"given": "Sumeera",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "R & D Hamdard Laboratories (Medicine Division) Ghaziabad Uttar Pradesh India"
}
],
"family": "Rana",
"given": "Naushad Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Arora",
"given": "Mohini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Hematology Dr Dang's Lab Pvt Ltd New Delhi India"
}
],
"family": "Jain",
"given": "Sonal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Community Medicine Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Islam",
"given": "Farzana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "R & D Hamdard Laboratories (Medicine Division) Ghaziabad Uttar Pradesh India"
}
],
"family": "Agarwal",
"given": "Shashank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Community Medicine Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"family": "Kashyap",
"given": "Varun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "R & D Hamdard Laboratories (Medicine Division) Ghaziabad Uttar Pradesh India"
}
],
"family": "Joshi",
"given": "Santosh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "R & D Hamdard Laboratories (Medicine Division) Ghaziabad Uttar Pradesh India"
}
],
"family": "Mueed",
"given": "Asad",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5235-5351",
"affiliation": [
{
"name": "Department of Microbiology Hamdard Institute of Medical Sciences and Research and associated HAHC Hospital New Delhi India"
}
],
"authenticated-orcid": false,
"family": "Dudeja",
"given": "Mridu",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T04:58:30Z",
"timestamp": 1657083510000
},
"deposited": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T04:58:44Z",
"timestamp": 1657083524000
},
"indexed": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T05:42:08Z",
"timestamp": 1657086128181
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
5
]
]
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
5
]
],
"date-time": "2022-07-05T00:00:00Z",
"timestamp": 1656979200000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
5
]
],
"date-time": "2022-07-05T00:00:00Z",
"timestamp": 1656979200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7531",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7531",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7531",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
7,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/PTR.6024",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.30493/DLS.2020.225404",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.3389/fphar.2020.590598",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1038/s41598-020-74715-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.3390/nu12041181",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1016/j.dsx.2020.07.028",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.4103/pm.pm_583_17",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1056/nejme2020388",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"article-title": "Bioactive molecules from eucalyptus essential oil as potential inhibitors of COVID‐19 corona virus infection by molecular docking studies",
"author": "Dev Sharma A.",
"first-page": "29",
"journal-title": "Kragujevac Journal of Science",
"key": "e_1_2_11_10_1",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1016/j.jep.2013.04.040",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1002/PTR.2295",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/j.healthpol.2021.03.013",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"key": "e_1_2_11_14_1",
"unstructured": "Government of India M. of A. (2009).Unani Pharmacopoeial of India ‐(government of India)(Vol.6)."
},
{
"author": "Government of India, M. of A",
"key": "e_1_2_11_15_1",
"volume-title": "The Unani pharmacopoeia of India part ‐II volume ‐III (formulations)",
"year": "2016"
},
{
"key": "e_1_2_11_16_1",
"unstructured": "Hakeem Najmul Gani Rampuri. (2009).Khazain ul Advia(Vol.2)."
},
{
"DOI": "10.1128/msphere.00922-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1016/J.HELIYON.2021.E07240",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1016/S2665-9913(20)30390-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1016/j.phymed.2020.153361",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/j.curtheres.2020.100602",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1111/apm.12928",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.1080/07391102.2020.1852119",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"key": "e_1_2_11_24_1",
"unstructured": "Ministry of AYUSH. (2009).Unani Pharmacopoeia of India (Government of India) (Vol. 6)."
},
{
"key": "e_1_2_11_25_1",
"unstructured": "Ministry of AYUSH. (n.d.).The Unani Pharmacopoeia of India ‐ Part 1 Volume 1 (Vol. V1)."
},
{
"DOI": "10.26434/chemrxiv.11987475",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_26_1",
"unstructured": "Mohammadi N. &Shaghaghi N.(2020).Inhibitory effect of eight secondary metabolites from conventional medicinal plants on COVID‐19 virus protease by molecular docking analysis. ChemRxiv Preprint.https://doi.org/10.26434/CHEMRXIV.11987475.V1"
},
{
"key": "e_1_2_11_27_1",
"unstructured": "Mukherjee P. K. &Mao A. A.(2021).Compendium of antiviral medicinal plants of north East India."
},
{
"key": "e_1_2_11_28_1",
"unstructured": "National Centre for Disease Control Directorate General of Health Services & MoHFW G. (2020).The updated case definitions and contact‐categorisation. Retrieved fromhttps://ncdc.gov.in/WriteReadData/l892s/89568514191583491940.pdf"
},
{
"DOI": "10.1016/B978-0-12-814466-4.00009-4",
"author": "Ramos‐Tovar E.",
"doi-asserted-by": "crossref",
"first-page": "101",
"key": "e_1_2_11_29_1",
"volume-title": "Dietary interventions in liver disease: Foods, nutrients, and dietary supplements",
"year": "2019"
},
{
"DOI": "10.35772/ghm.2021.01010",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"author": "Saboo S.",
"key": "e_1_2_11_31_1",
"volume-title": "Intech Open",
"year": "2021"
},
{
"DOI": "10.1016/J.BIOPHA.2020.110267",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.1002/PTR.7228",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"DOI": "10.1186/S43094-021-00259-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"key": "e_1_2_11_35_1",
"unstructured": "Unani Pharmacopeia committee. Government of India. (2007).National Formulary of Unani Medicine (N.F.U.M.) (Vol. 1)."
},
{
"DOI": "10.1016/j.medmal.2020.03.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"key": "e_1_2_11_37_1",
"unstructured": "World Health Organization. (2021).India: WHO Coronavirus Disease (COVID‐19) Dashboard With Vaccination Data. Retrieved fromhttps://covid19.who.int/region/searo/country/in"
},
{
"key": "e_1_2_11_38_1",
"unstructured": "World Health Organization. (2021a).Weekly epidemiological update on COVID‐19 ‐ May 11 2021."
},
{
"key": "e_1_2_11_39_1",
"unstructured": "World Health Organization. (2021b).WHO advises that ivermectin only be used to treat COVID‐19 within clinical trials. Retrieved fromhttps://www.who.int/news-room/feature-stories/detail/who-advises-that-ivermectin-only-be-used-to-treat-covid-19-within-clinical-trials."
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7531"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Efficacy of polyherbal formulations for prevention of\n <scp>COVID</scp>\n ‐19 infection in high‐risk subjects: A randomized open‐label controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}