Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciae408, Aug 2024
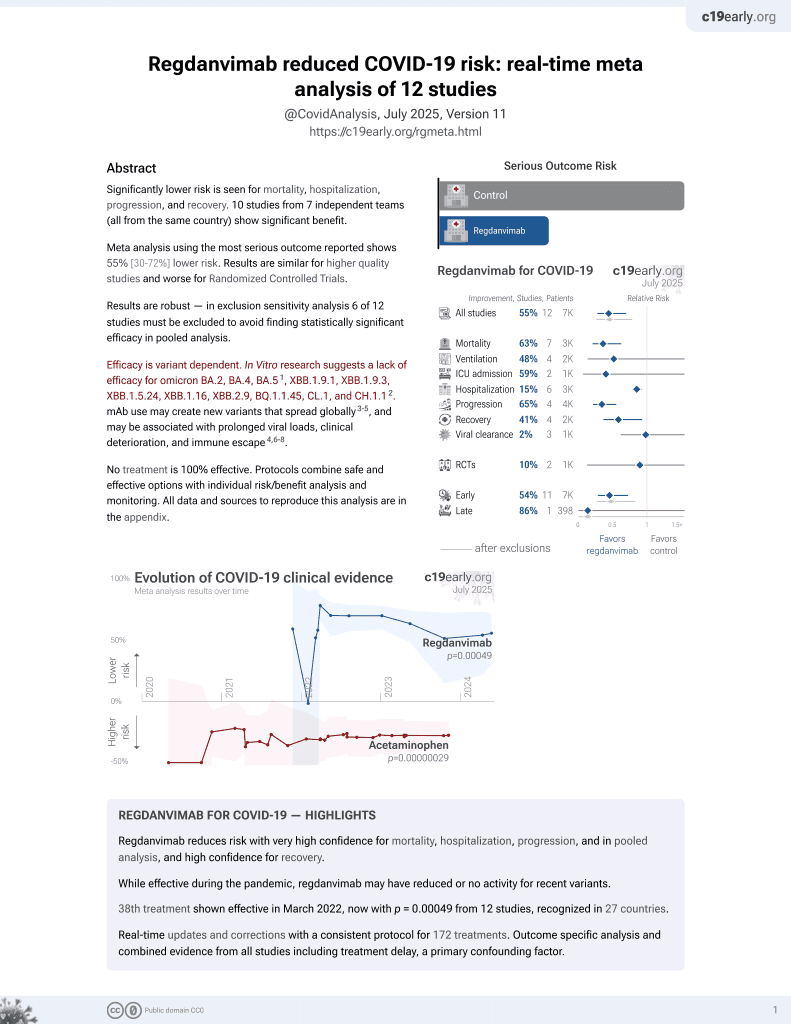
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Review arguing against use of single monoclonal antibodies for COVID-19 treatment, particularly in immunosuppressed patients, due to the risk of rapidly selecting for resistant viral variants. Authors suggest that while monoclonal antibodies may be justified for pre-exposure prophylaxis, their use in therapy poses potential risks to patients, society, and the future viability of monoclonal antibody treatments.
Review covers casirivimab/imdevimab, bamlanivimab/etesevimab, tixagevimab/cilgavimab, bebtelovimab, sotrovimab, adintrevimab, regdanvimab, amubarvimab, BMS mAbs, and pemivibart.
Casadevall et al., 8 Aug 2024, peer-reviewed, 4 authors.
DOI record:
{
"DOI": "10.1093/cid/ciae408",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciae408",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>The COVID-19 pandemic saw the largest deployment of monoclonal antibodies (mAbs) for an infectious disease in history. mAbs to SARS-CoV-2 spike protein proved safe and were initially effective for COVID-19 therapy, but each was defeated by continued SARS-CoV-2 evolution, leading to their withdrawal. This was a setback for people with impaired immunity who cannot mount an effective antibody response to SARS-CoV-2 and often cannot clear the virus. New mAbs have now been developed for pre-exposure prophylaxis (PreEP) in immunosuppressed people. Here we argue that while mAb use for PreEP is justified, single mAbs should not be used for COVID-19 therapy. In contrast to PreEP where the viral inoculum is small, immunosuppressed people with COVID-19 have large viral burden that can harbor mAb-escape variants that single-agent mAb treatments can rapidly select for resistance. Selection of mAb-escape variants has potential risks for patients, society and the feasibility of mAb therapy itself.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9402-9167",
"affiliation": [
{
"name": "Department of Molecular Microbiology and Immunology, Johns Hopkins School of Public Health , Baltimore, MD"
}
],
"authenticated-orcid": false,
"family": "Casadevall",
"given": "Arturo",
"sequence": "first"
},
{
"affiliation": [
{
"name": "North-Western Tuscany Blood Bank, Pisa University Hospital , Pisa , Italy"
}
],
"family": "Focosi",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Divison of Infectious Diseases, Albert Einstein College of Medicine , Bronx, NY"
}
],
"family": "Pirofski",
"given": "Liise-anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Johns Hopkins School of Medicine , Baltimore, MD"
}
],
"family": "Shoham",
"given": "Shmuel",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
8
]
],
"date-time": "2024-08-08T19:03:44Z",
"timestamp": 1723143824000
},
"deposited": {
"date-parts": [
[
2024,
8,
8
]
],
"date-time": "2024-08-08T19:03:45Z",
"timestamp": 1723143825000
},
"indexed": {
"date-parts": [
[
2024,
8,
9
]
],
"date-time": "2024-08-09T00:22:51Z",
"timestamp": 1723162971034
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
8,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
8,
8
]
],
"date-time": "2024-08-08T00:00:00Z",
"timestamp": 1723075200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae408/58775511/ciae408.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciae408/58775511/ciae408.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2024,
8,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
8,
8
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciae408/7729969"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Single monoclonal antibodies should not be used for COVID-19 therapy: a call for antiviral stewardship",
"type": "journal-article"
}
casadevall