Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1809, Jan 2026
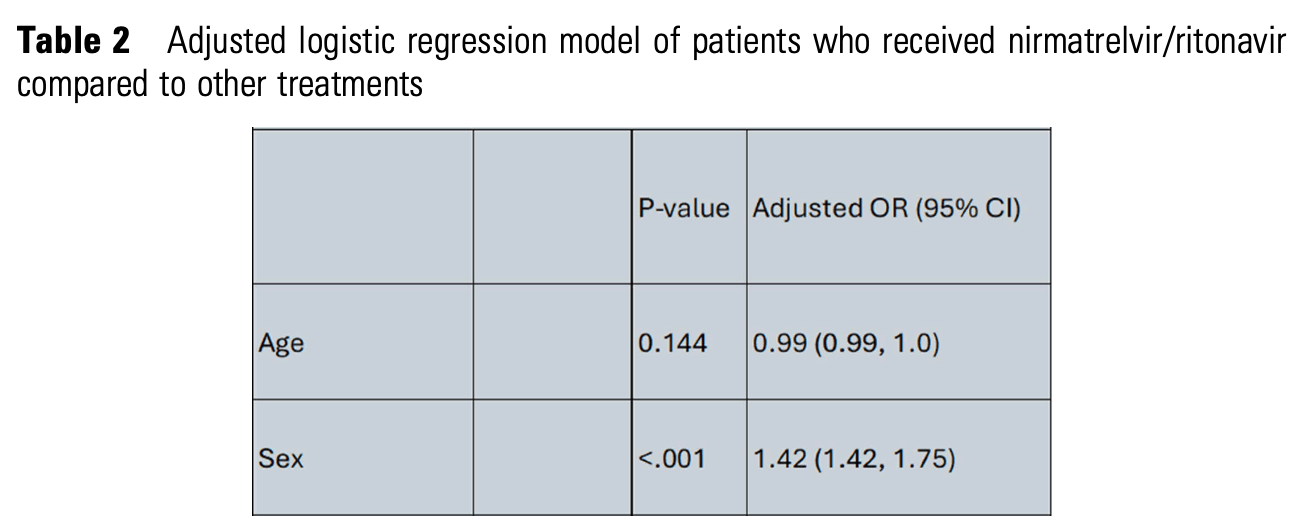
Retrospective 3,498 patients receiving COVID-19 antivirals showing that female patients were significantly more likely to receive treatment.
Studies show that female patients are significantly more likely to be "health-conscious", for example researching conditions and taking other non-prescription treatments, lifestyle changes, etc., which may significantly confound observational studies.
Campion et al., 11 Jan 2026, retrospective, peer-reviewed, 3 authors.
P-1633. Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study
2 ) support received during the baseline, in the first 2 days. 2 upon admission: renal 0.79 (0.69, 0.91), hepatic 0.73 (0.56, 0.95). Hepatic patients who did not require supplemental O 2 also had significantly lower mortality risk: 0.55 (0.34, 0.89). Mortality risk reduction in renal patients not requiring supplemental O 2 was not statistically significant: 0.87 (0.68, 1.11). 2 .
DOI record:
{
"DOI": "10.1093/ofid/ofaf695.1809",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofaf695.1809",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>COVID-19 has caused significant morbidity and mortality which disproportionately impacted racial and ethnic minorities. The introduction of nirmatrelvir/ritonavir (n/r) has introduced an oral option for treatment, but its use was limited by drug-drug interactions. In this study, we aim to evaluate the factors associated with the prescription of nirmatrelvir/ritonavir (n/r) compared to other COVID-19 antivirals in a healthcare system in Eastern Massachusetts.Table 1Baseline demographic and clinical characteristics of patients who received nirmatrelvir/ritonavir compared to other treatmentsTable 2Adjusted logistic regression model of patients who received nirmatrelvir/ritonavir compared to other treatments</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This is a retrospective study including adult patients with a COVID-19 diagnosis. The Vizient Clinical Database, which captures patient-level data, was used to identify encounters between Oct/2022 and Jul/2024. The primary study outcome was evaluating patients who received COVID-19 antivirals with molnupiravir, remdesivir or combination of multiple agents compared to those who received n/r. Demographic data including Vizent vulnerability index (VVI) were collected. VVI score ranges between -3 to 3, higher values indicates increased vulnerability. Patient characteristics by treatment group were presented as counts and percentages for categorical variables and medians with interquartile ranges for continuous variables. The primary analysis was a logistic regression model evaluating factors associated with n/r prescription.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We identified 3498 who received COVID-19 antivirals. Most patients received n/r for treatment (n = 2106, 60.2%). Patients’ characteristics are outlined in Table 1. In the multivariate model female sex, outpatient encounters, higher VVI (more socially vulnerable) and non-White race remained significantly associated with increased odds of being prescribed n/r (Table 2). Inpatient encounter and Medicare coverage were associated with reduced odds of getting n/r as compared to patients seen in the ED.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Prescriptions for n/r is different across the healthcare system. N/r was found to be prescribed more in the outpatient and ED setting which is unsurprising based upon its oral dosage form. However, patients with lower socioeconomic status based upon the VVI and non-White were more likely to be prescribed n/r. These findings highlight demographic and socioeconomic differences in the prescribing patterns of COVID-19 antiviral therapies.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>All Authors: No reported disclosures</jats:p>\n </jats:sec>",
"article-number": "ofaf695.1809",
"author": [
{
"affiliation": [
{
"name": "Tufts Medical Center , Boston, Massachusetts"
}
],
"family": "Campion",
"given": "Maureen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Tufts Medical Center , Boston, Massachusetts"
}
],
"family": "Alsoubani",
"given": "Majd",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Tufts Medical Center , Boston, Massachusetts"
}
],
"family": "Andujar Vazquez",
"given": "Gabriela",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T07:28:51Z",
"timestamp": 1768202931000
},
"deposited": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T07:41:03Z",
"timestamp": 1768203663000
},
"indexed": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T13:32:56Z",
"timestamp": 1768224776830,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "Supplement_1",
"issued": {
"date-parts": [
[
2026,
1
]
]
},
"journal-issue": {
"issue": "Supplement_1",
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2026,
1,
12
]
],
"date-time": "2026-01-12T00:00:00Z",
"timestamp": 1768176000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.1809/66354793/ofaf695.1809.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/13/Supplement_1/ofaf695.1809/66354793/ofaf695.1809.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2026,
1
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
11
]
]
},
"published-other": {
"date-parts": [
[
2026,
1
]
]
},
"published-print": {
"date-parts": [
[
2026,
1,
11
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofaf695.1809/8421988"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "P-1633. Disparities in the Use of nirmatrelvir/ritonavir for COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"volume": "13"
}