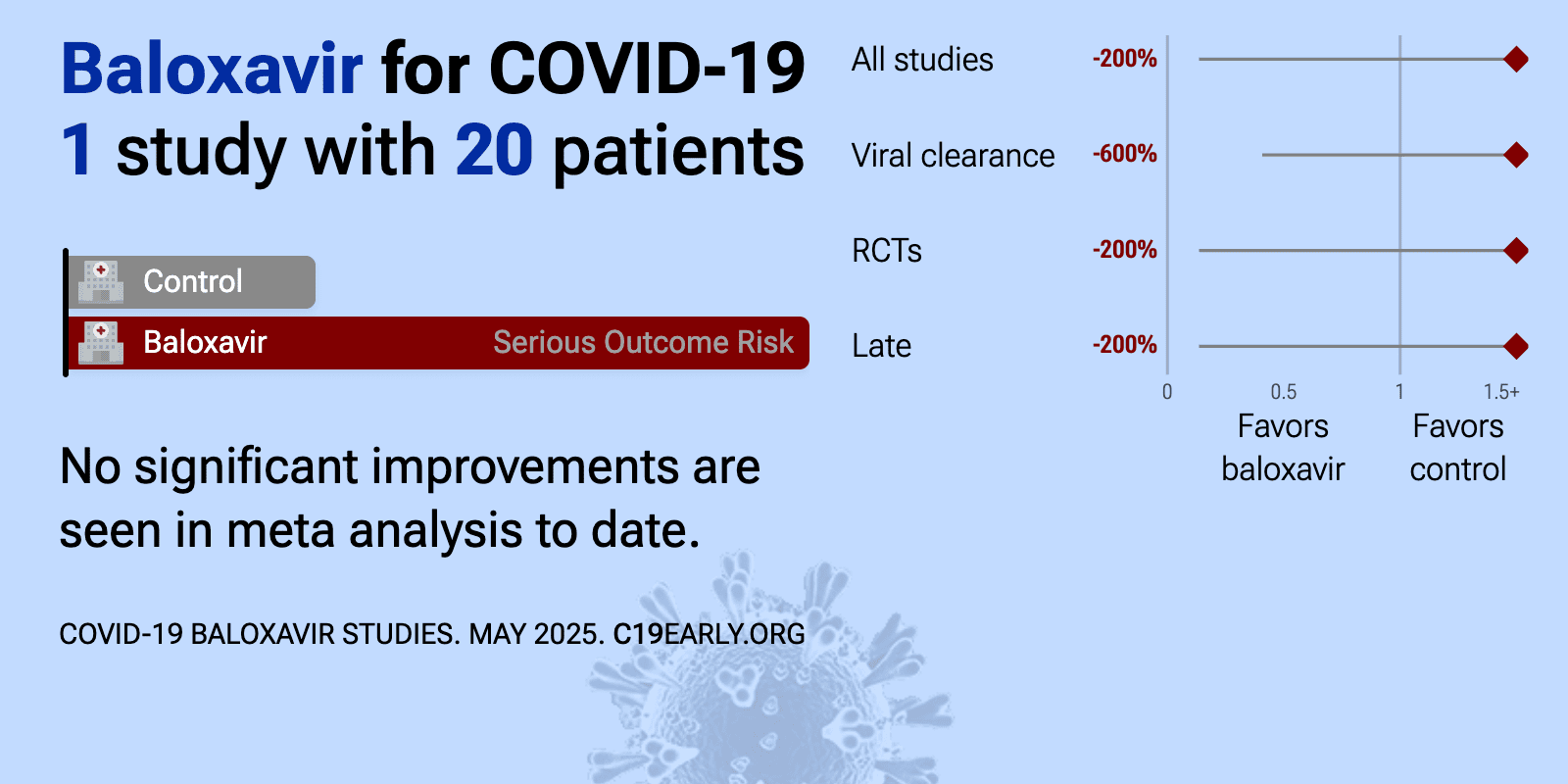
Oct 25 2020 |
et al., European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2020.105631 | Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial |
| 20% improved recovery (p=1) and 600% worse viral clearance (p=0.21). Small late stage RCT with 10 favipiravir, 10 baloxavir marboxil, and 10 control patients in China, showing no significant differences. The single-dose influenza regimen produced plasma levels far below the SARS-CoV-2 EC50. | ||