SARS-CoV-2 viral dynamics in a placebo-controlled phase 2 study of patients infected with the SARS-CoV-2 Omicron variant and treated with pomotrelvir
et al., Microbiology Spectrum, doi:10.1128/spectrum.02980-23, NCT05543707, Feb 2024
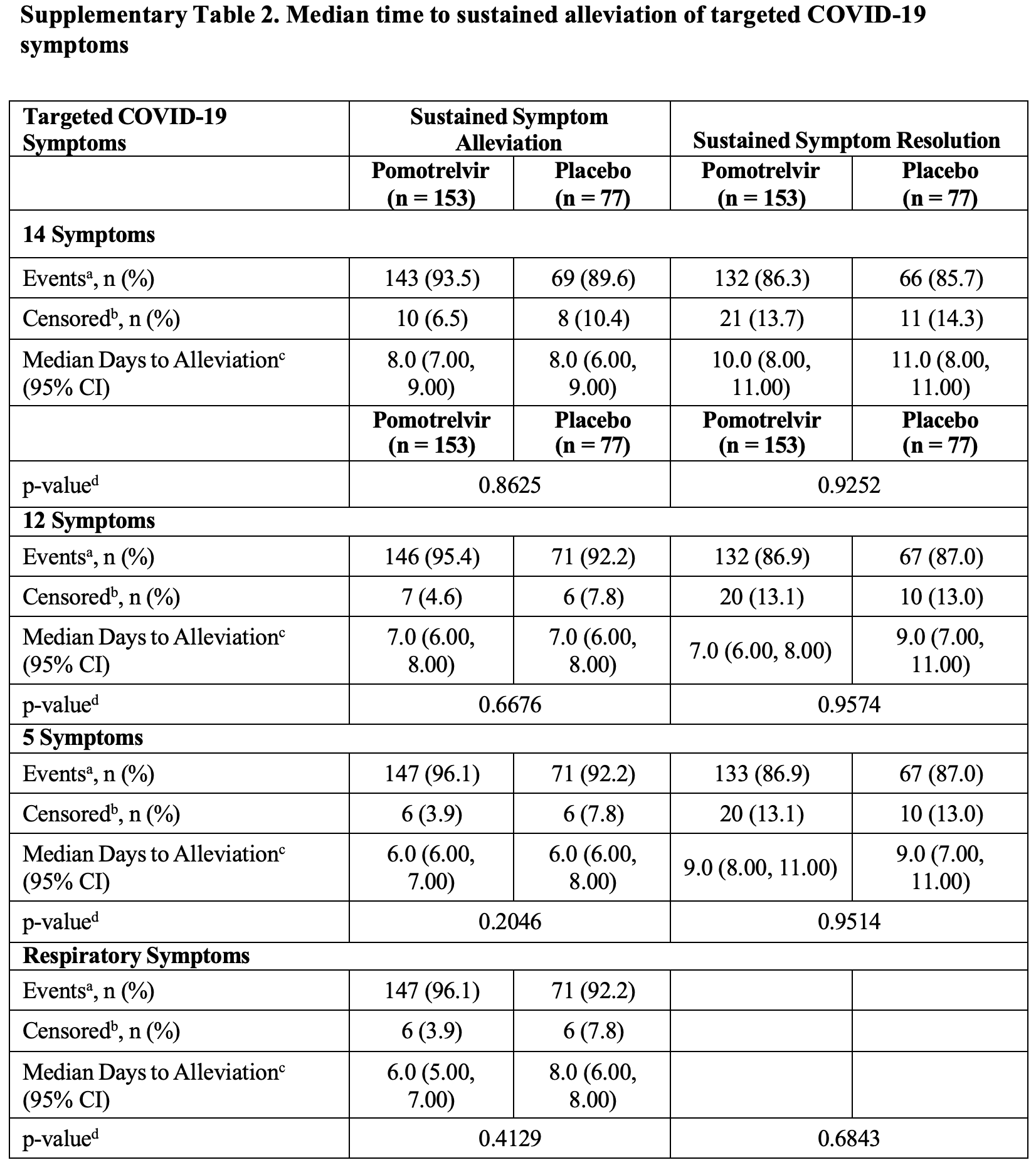
RCT 242 symptomatic outpatients showing no significant difference in viral clearance or symptom resolution with pomotrelvir treatment initiated within 5 days of symptom onset. Based on the description provided it is not clear why the number of censored patients changes in the treatment group between the 5, 12, and 14 symptom results. The event count and percentage reported for the 12 symptom result do not match.
Authors also compare viral clearance based on infectious virus assay (IVA), RAT, and PCR, which shows that PCR/RAT results are often positive when IVA shows no infectious virus.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of no recovery, 0.7% higher, RR 1.01, p = 1.00, treatment 20 of 153 (13.1%), control 10 of 77 (13.0%), sustained resolution, 5 main symptoms, day 28.
|
|
risk of no recovery, 5.7% higher, RR 1.06, p = 1.00, treatment 21 of 153 (13.7%), control 10 of 77 (13.0%), sustained resolution, 12 symptoms, day 28.
|
|
risk of no recovery, 3.9% lower, RR 0.96, p = 1.00, treatment 21 of 153 (13.7%), control 11 of 77 (14.3%), NNT 179, sustained resolution, 14 symptoms, day 28.
|
|
rebound, 252.3% higher, RR 3.52, p = 0.10, treatment 14 of 153 (9.2%), control 2 of 77 (2.6%), day 28.
|
|
risk of no viral clearance, 39.3% higher, RR 1.39, p = 1.00, treatment 3 of 84 (3.6%), control 1 of 39 (2.6%), day 5.
|
|
risk of no viral clearance, 14.8% higher, RR 1.15, p = 0.56, treatment 47 of 84 (56.0%), control 19 of 39 (48.7%), day 3, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Borroto-Esoda et al., 6 Feb 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 43.0, 6 authors, study period January 2021 - May 2021, trial NCT05543707 (history).
Contact: ann.kwong@kwongpharmaconsulting.com.
SARS-CoV-2 viral dynamics in a placebo-controlled phase 2 study of patients infected with the SARS-CoV-2 Omicron variant and treated with pomotrelvir
Microbiology Spectrum, doi:10.1128/spectrum.02980-23
Current guidelines recommend that individuals with moderate COVID-19 disease isolate for 5 days after the first appearance of symptoms or a positive SARS-CoV-2 test. It would be useful to understand the time course of infectious virus production and its correlation with virus detection using a rapid antigen test (RAT) or quantitative reverse transcriptase (qRT)-PCR. In a phase 2 study, 242 vaccinated patients with COVID-19 and at low risk for progression to severe disease initiated 5 days of treatment with pomotrelvir (PBI-0451, a SARS-CoV-2 main protease inhibitor) or placebo within 5 days after symptom onset. The primary endpoint, the proportion of subjects with SARS-CoV-2 viral titers below the limit of detection on Day 3 of treatment in the pomotrelvir versus placebo groups, was not met. No between-group differences in SARS-CoV-2 clearance or symptom resolution or alleviation were observed. Additional analyses evaluated the dynamics of SARS-CoV-2 replication in mid-turbinate nasal swabs and saliva samples using infectious virus assay (IVA), RAT, and qRT-PCR. SARS-CoV-2 cleared rapidly, with negative results first determined by IVA (TCID 50 below the limit of detection), followed by the RAT (negative for SARS-CoV-2 N antigen), and qRT-PCR (RNA below the limit of detection), which suggests that delayed initiation of treatment (up to 5 days after symptom onset) may have contributed to the lack of treatment response. Symptom resolution lagged behind viral clearance assessed by IVA and RAT. These data support reliance on a negative RAT to determine when an individual is no longer producing infectious virus and may end isolation. IMPORTANCE A phase 2 double-blind, placebo-controlled study was performed evaluating pomotrelvir, a SARS-CoV-2 Mpro inhibitor, compared with placebo in 242 non-hospitalized, vaccinated, symptomatic adults with COVID-19 (Omicron). No improvement in the decrease of viral replication or relief of symptoms was observed between the two groups when treatment was initiated ≥3 days after symptom onset. These results suggest that future COVID-19 antiviral studies using a similar patient population may need to initiate treatment earlier, like influenza studies. This is the first study to prospectively evaluate SARS-CoV-2 viral dynamics and the time to viral clearance in a significant number of patients using concurrently obtained results from an infectious virus assay, a rapid antigen test (RAT), and a qRT-PCR assay over a 15-day time course. These results suggest that a negative RAT assay is a good indicator of loss of infectious virus and the ability to return to normal activities.
MATERIALS AND METHODS
Study design A phase multicenter, randomized, double-blind, placebo-controlled study that the antiviral activity, clinical efficacy, and safety of orally administered pomotrelvir compared with placebo.
Patients Patients were non-hospitalized, symptomatic males and females from 18 to <65 years old with COVID-19, who were not at high risk of progressing to severe disease, with onset of COVID-19 symptoms within 5 days prior to randomization, a positive SARS-CoV-2 test within 24 hours prior to randomization, and at least two symptoms of acute SARS-CoV-2 infection at randomization. Patients had a primary COVID-19 vaccination series (and any booster) at least 3 months prior to randomization.
Randomization and blinding Patients were randomized 2:1 to the pomotrelvir or placebo treatment group and assigned a unique subject number via an interactive voice and web response system. Randomization was stratified based on SARS-CoV-2 positive direct test diagnosis ≤3 days versus >3-5 days from the first onset of COVID-19 symptom(s) and if patients received primary vaccination series alone versus any COVID-19 booster doses. Patients, investigators, and all internal and external personnel directly involved in the conduct of the study were blinded to treatment assignment.
Treatment and procedures Following randomization on Day 1, subjects initiated dosing with either pomotrelvir 700 mg (2× 350 mg tablets twice daily for a total daily dose of 1,400 mg) or..
References
Anderson, Caubel, Rusnak, Investigators, Nirmatrel vir-ritonavir and viral load rebound in COVID-19, N Engl J Med, doi:10.1128/aac.00697-22
Chen, Wang, Gilby, Wei, Omicron variant (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance, J Chem Inf Model, doi:10.1016/S0140-6736(22)00462-7
Chuvt, Schwartzng, Donnellymap, Chuey, Soto et al., Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection, JAMA Intern Med, doi:10.1016/S0140-6736(22)00462-7
Fischer, Eron, Holman, Cohen, Fang et al., A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med, doi:10.1126/scitranslmed.abl7430
Hakki, Zhou, Jonnerby, Singanayagam, Barnett et al., Onset and window of SARS-CoV-2 infectious ness and temporal correlation with symptom onset: a prospective, longitudinal, community cohort study, Lancet Respir Med, doi:10.1016/S2213-2600(22)00226-0
Hayden, Sugaya, Hirotsu, Lee, De Jong et al., Baloxavir marboxil for uncomplicated influenza in adults and adolescents, N Engl J Med, doi:10.1056/NEJMoa1716197
Jayamohan, Lambert, Sant, Jafek, Patel et al., SARS-CoV-2 pandemic: a review of molecular diagnostic tools including sample collection and commercial response with associated advantages and limitations, Anal Bioanal Chem, doi:10.1007/s00216-020-02958-1
Kearneybp, Wolfgangghi, Turnquist, Marshall, Pruijssers et al., an orally administered 3CL protease inhibitor of SARS-CoV-2 for COVID-19, doi:10.1016/S0140-6736(22)00462-7
Kirby, Riedel, Dutta, Arnaout, Cheng et al., SARS-CoV-2 antigen test predict infectivity based on viral culture: comparison of antigen, PCR viral load, and viral culture testing on a large sample cohort, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.07.010
Kompaniyets, Pennington, Goodman, Rosenblum, Belay et al., Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020, Prev Chronic Dis, doi:10.5888/pcd18.210123
Lane, Hunter, Lee, Hyman, Bross et al., Clinical characteristics and symptom duration among outpatients with COVID-19, Am J Infect Control, doi:10.1128/aac.00697-22
Lee, Herigon, Benedetti, Pollock, Denkinger, Performance of saliva, oropharyngeal swabs, and nasal swabs for SARS-CoV-2 molecular detection: a systematic review and meta-analysis, J Clin Microbiol, doi:10.1128/JCM.02881-20
Mukae, Yotsuyanagi, Ohmagari, Doi, Imamura et al., A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-tomoderate COVD-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2A part, Antimicrob Agents Chemother, doi:10.1128/aac.00697-22
Mukae, Yotsuyanagi, Ohmagari, Doi, Sakaguchi et al., Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study, Clin Infect Dis, doi:10.1128/aac.00697-22
Navero-Castillejos, Casals-Pascual, Narváez, Cuesta, Hurtado et al., Diagnostic perform ance of six rapid antigen tests for SARS-CoV-2, Microbiol Spectr, doi:10.1128/spectrum.02351-21
Nyberg, Ferguson, Nash, Webster, Flaxman et al., Comparative analysis of the risks of hospitalization and death associated with SARS-CoV-2 Omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: A cohort study, The Lancet, doi:10.1016/S0140-6736(22)00462-7
Petersen, Kongsstovu, Eliasen, Larsen, Hansen et al., Clinical characteristics of the Omicron variant -results from a nationwide symptoms survey in the Faroe Islands, Int J Infect Dis, doi:10.1016/S0140-6736(22)00462-7
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Escapes of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1016/S0140-6736(22)00462-7
Sia, Yan, Chin, Fung, Choy et al., Pathogenesis and transmission of SARS-CoV-2 in golden hamsters, Nature, doi:10.1038/s41586-020-2342-5
Tan, Kwan, Rodríguez-Barraquer, Singer, Park et al., Infectiousness of SARS-CoV-2 breakthrough infections and Reinfections during the Omicron wave, Nat Med, doi:10.1016/S0140-6736(22)00462-7
Viana, Moyo, Amoako, Tegally, Scheepers et al., Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in Southern Africa, Nature, doi:10.1016/S0140-6736(22)00462-7
Wang, Berger, Kaelber, Davis, Volkow et al., COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after Omicron emerged in the US, Infectious diseases (except HIV/AIDS, doi:10.1016/S0140-6736(22)00462-7
DOI record:
{
"DOI": "10.1128/spectrum.02980-23",
"ISSN": [
"2165-0497"
],
"URL": "http://dx.doi.org/10.1128/spectrum.02980-23",
"abstract": "<jats:title>ABSTRACT</jats:title>\n <jats:p>\n Current guidelines recommend that individuals with moderate COVID-19 disease isolate for 5 days after the first appearance of symptoms or a positive SARS-CoV-2 test. It would be useful to understand the time course of infectious virus production and its correlation with virus detection using a rapid antigen test (RAT) or quantitative reverse transcriptase (qRT)-PCR. In a phase 2 study, 242 vaccinated patients with COVID-19 and at low risk for progression to severe disease initiated 5 days of treatment with pomotrelvir (PBI-0451, a SARS-CoV-2 main protease inhibitor) or placebo within 5 days after symptom onset. The primary endpoint, the proportion of subjects with SARS-CoV-2 viral titers below the limit of detection on Day 3 of treatment in the pomotrelvir versus placebo groups, was not met. No between-group differences in SARS-CoV-2 clearance or symptom resolution or alleviation were observed. Additional analyses evaluated the dynamics of SARS-CoV-2 replication in mid-turbinate nasal swabs and saliva samples using infectious virus assay (IVA), RAT, and qRT-PCR. SARS-CoV-2 cleared rapidly, with negative results first determined by IVA (TCID\n <jats:sub>50</jats:sub>\n below the limit of detection), followed by the RAT (negative for SARS-CoV-2 N antigen), and qRT-PCR (RNA below the limit of detection), which suggests that delayed initiation of treatment (up to 5 days after symptom onset) may have contributed to the lack of treatment response. Symptom resolution lagged behind viral clearance assessed by IVA and RAT. These data support reliance on a negative RAT to determine when an individual is no longer producing infectious virus and may end isolation.\n </jats:p>\n <jats:sec>\n <jats:title>IMPORTANCE</jats:title>\n <jats:p>A phase 2 double-blind, placebo-controlled study was performed evaluating pomotrelvir, a SARS-CoV-2 Mpro inhibitor, compared with placebo in 242 non-hospitalized, vaccinated, symptomatic adults with COVID-19 (Omicron). No improvement in the decrease of viral replication or relief of symptoms was observed between the two groups when treatment was initiated ≥3 days after symptom onset. These results suggest that future COVID-19 antiviral studies using a similar patient population may need to initiate treatment earlier, like influenza studies. This is the first study to prospectively evaluate SARS-CoV-2 viral dynamics and the time to viral clearance in a significant number of patients using concurrently obtained results from an infectious virus assay, a rapid antigen test (RAT), and a qRT-PCR assay over a 15-day time course. These results suggest that a negative RAT assay is a good indicator of loss of infectious virus and the ability to return to normal activities.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.1128/spectrum.02980-23"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-07-31"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-11-24"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-01-10"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3909-6578",
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"authenticated-orcid": false,
"family": "Borroto-Esoda",
"given": "Katyna",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"family": "Wilfret",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"family": "Tong",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"family": "Plummer",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"family": "Kearney",
"given": "Brian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8460-9857",
"affiliation": [
{
"name": "Pardes BioSciences Inc., Carlsbad, California, USA"
}
],
"authenticated-orcid": false,
"family": "Kwong",
"given": "Ann D.",
"sequence": "additional"
}
],
"container-title": "Microbiology Spectrum",
"container-title-short": "Microbiol Spectr",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2024,
1,
10
]
],
"date-time": "2024-01-10T14:01:36Z",
"timestamp": 1704895296000
},
"deposited": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T14:06:44Z",
"timestamp": 1707228404000
},
"editor": [
{
"affiliation": [],
"family": "Piantadosi",
"given": "Anne",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
16
]
],
"date-time": "2024-05-16T10:09:00Z",
"timestamp": 1715854140678
},
"is-referenced-by-count": 1,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
2,
6
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2024,
2,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T00:00:00Z",
"timestamp": 1707177600000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
6
]
],
"date-time": "2024-02-06T00:00:00Z",
"timestamp": 1707177600000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/spectrum.02980-23",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/spectrum.02980-23",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2024,
2,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
2,
6
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"DOI": "10.1101/2022.02.21.22271300",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_2_2",
"unstructured": "Wang L Berger NA Kaelber DC Davis PB Volkow ND Xu R. 2022. COVID infection rates clinical outcomes and racial/ethnic and gender disparities before and after Omicron emerged in the US. Infectious diseases (except HIV/AIDS):2022.02.21.22271300. doi:10.1101/2022.02.21.22271300"
},
{
"DOI": "10.1038/s41586-022-04411-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.1021/acs.jcim.1c01451",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1038/s41591-022-02138-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1016/j.ijid.2022.07.005",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.5888/pcd18.210123",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_2"
},
{
"author": "Kearney BP",
"key": "e_1_3_4_10_2",
"unstructured": "KearneyBP, PlummerA, WolfgangGHI, Turnquist D, Marshall MR, Pruijssers A, Stevens LJ, Hughes TM, Kook S, Denison M, Schwabe C, Lopatin U, Arnold LD. 2022. Conference on retroviruses and opportunistic infections, poster 00470. PBI-0451: an orally administered 3CL protease inhibitor of SARS-CoV-2 for COVID-19",
"volume-title": "PBI-0451: an orally administered 3CL protease inhibitor of SARS-CoV-2 for COVID-19",
"year": "2022"
},
{
"DOI": "10.1007/s00216-020-02958-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.1128/spectrum.02351-21",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1001/jamainternmed.2022.1827",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1016/j.cmi.2022.07.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1093/cid/ciac933",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1056/NEJMc2205944",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1016/j.ajic.2021.10.039",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1128/JCM.02881-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1038/s41586-020-2342-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.1016/S2213-2600(22)00226-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1128/aac.00697-22",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.1056/NEJMoa1716197",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/spectrum.02980-23"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "SARS-CoV-2 viral dynamics in a placebo-controlled phase 2 study of patients infected with the SARS-CoV-2 Omicron variant and treated with pomotrelvir",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "12"
}