Lessons from the PROTECT-CH COVID-19 platform trial in care homes
et al., Health Technology Assessment, doi:10.3310/MTRS8833, Apr 2025
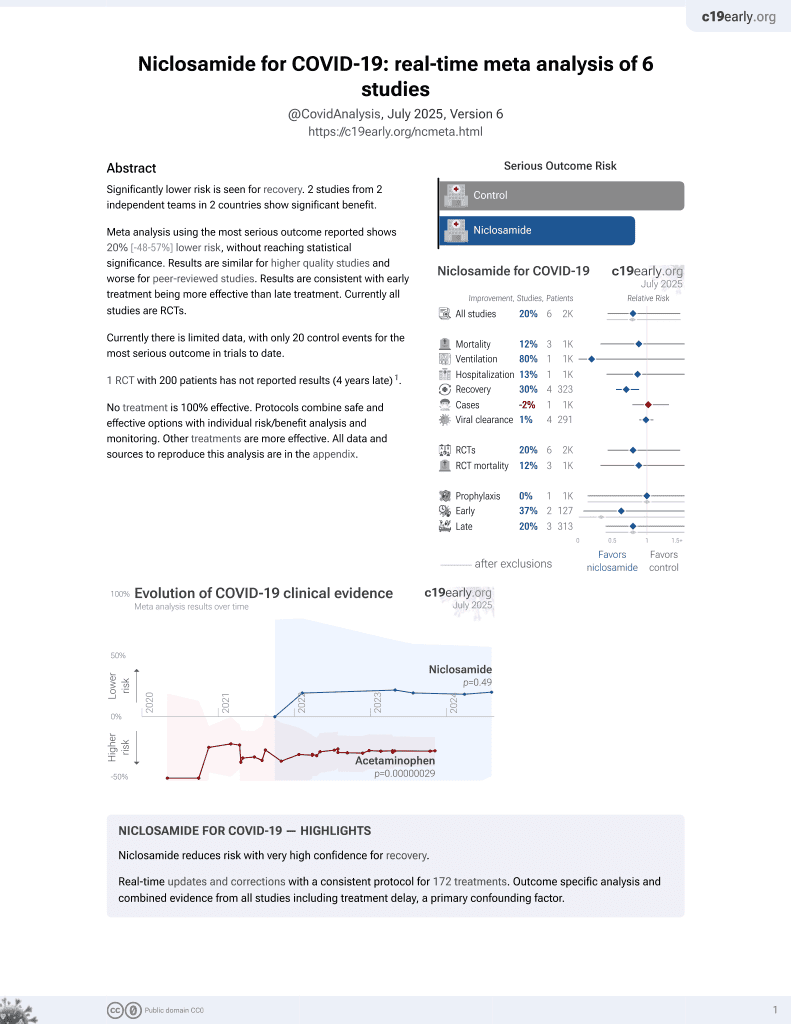
56th treatment shown to reduce risk in
August 2025, now with p = 0.0069 from 7 studies.
Lower risk for recovery.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Discussion of a planned COVID-19 platform trial (PROTECT-CH) in care homes that failed to start recruitment. The trial was designed to test prophylactic antiviral interventions (initially ciclesonide and niclosamide) to reduce SARS-CoV-2 transmission and disease severity in care home residents.
Despite developing the infrastructure and identifying 300 care homes, multiple logistical challenges prevented implementation, including issues with drug contracting, complex care home governance structures, difficulties coordinating general practitioners, research nurse shortages, insurance complications, and regulatory hurdles across four UK nations.
Bath et al., 30 Apr 2025, prospective, placebo-controlled, United Kingdom, peer-reviewed, 26 authors.
Contact: philip.bath@nottingham.ac.uk.
Lessons from the PROTECT-CH COVID-19 platform trial in care homes
Health Technology Assessment, doi:10.3310/mtrs8833
Background: Coronavirus disease-2019 was associated with significant mortality and morbidity in care homes in 2020-1. Repurposed antiviral drugs might reduce morbidity and mortality through reducing viral transmission, infection, replication and inflammation. We aimed to compare the safety and efficacy of potential antiviral drugs in care home residents.
Authorship The PROTECT-CH trial did not contract with any care homes, GPs or PIs, so there are no other authors to be listed here.
Eligibility criteria
Care home eligibility at trial entry
Inclusion criteria • Location: UK care homes for older people, with and without nursing. • Size: ≥ 20 beds in the care home in total.
Exclusion criteria • CQC quality rating as inadequate, or equivalent in devolved administrations.
Care home eligibility at treatment phase
Exclusion criteria • Positive PCR or lateral flow test (or equivalent) for SARS-CoV-2 in any resident and/or staff within previous 4 weeks.
Resident eligibility at trial entry
Inclusion criteria • Resident in a care home. • Age ≥ 65 years. • Able to give informed consent for participation or a personal legal representative has been identified who can give consent if resident lacks capacity.
Exclusion criteria • Identified by care home staff to have entered endstage palliative care. • Resident in care home for short-term respite care. • Resident's GP is unable to support their involvement in the trial.
Resident eligibility at treatment phase
Exclusion criteria • Currently taking all of the trial interventions. • Contraindication to all trial interventions -see protocol's IMP below. • In treatment phase of another COVID-19 prevention or treatment trial.
References
Abdulamir, Gorial, Saadi, Maulood, Hashim et al., A randomised controlled trial of effectiveness and safety of niclosamide as add on therapy to the standard of care measures in COVID-19 management, Ann Med Surg
Adam L Gordon, Funding acquisition (co-lead), Methodology (equal), Project administration (equal), Resources (equal), Supervision (equal)
Amani, Akbarzadeh, Amani, Shabestan, Khorramnia et al., Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis, J Med Virol
Angus, Derde, Al-Beidh, Annane, Arabi et al., Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial, JAMA
Backer, Sjobring, Sonne, Weiss, Hostrup et al., A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19, Lancet Reg Health Eur
Baden, Sahly, Essink, Kotloff, Frey et al., Efficacy and safety DOI: 10.3310/MTRS8833 Health Technology Assessment 2025 22 NIHR Journals Library www.journalslibrary.nihr.ac.uk of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med
Bath, Ball, Boyd, Gage, Glover et al., Prophylactic treatment of COVID-19 in care homes trial (PROTECT-CH), medRxiv, doi:10.1101/2022.08.29.22279359
Bath, Coleman, Gordon, Lim, Webb, Nitric oxide for the prevention and treatment of viral, bacterial, protozoal and fungal infections, Res
Bath, Geeganage, Gray, Collier, Pocock, Optimising the analysis of stroke prevention trials: converting dichotomous vascular outcomes into ordinal measures, Stroke
Bath, Geeganage, Gray, Collier, Pocock, Use of ordinal outcomes in vascular prevention trials: comparison with binary outcomes in published stroke trials, Stroke
Bath, Geeganage, Gray, Ordinal reanalysis of the SHEP trial, Stroke
Bath, Gray, Collier, Pocock, Carpenter, Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials, Stroke
Bath, Lees, Schellinger, Altman, Bland et al., European Stroke Organisation Outcomes Working Group. Statistical analysis of the primary outcome in acute stroke trials, Stroke
Bath, Skinner, Bath, Woodhouse, Korovesi et al., for BEET-Winter Investigators. Dietary nitrate supplementation for preventing and reducing the severity of winter infections, including COVID-19, in care homes (BEET-Winter): a randomised placebo-controlled feasibility trial, Eur Geriatr Med
Bernal, Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Study Group. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Blueprint, Novel Coronavirus -COVID-19 Therapeutic Trial Synopsis
Brodin, Tornhammar, Ueda, Krifors, Westerlund et al., Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19), BMJ Open
Brunaugh, Seo, Warnken, Ding, Seo et al., Broad-spectrum, patient-adaptable inhaled niclosamide-lysozyme particles are efficacious against coronaviruses in lethal murine infection models, bioRxiv
Burton, Bayne, Evans, Garbe, Gorman et al., Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK, Lancet Healthy Longev
Burton, Reid, Gribben, Caldwell, Clark et al., Impact of COVID-19 on care-home mortality and life expectancy in Scotland, Age Ageing
Butler, Hobbs, Gbinigie, Rahman, Hayward et al., Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Buyse, George, Evans, Geller, Ranstam et al., The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials, Stat Med
Campbell, Piaggio, Elbourne, Altman, Group, statement: extension to cluster randomised trials, BMJ
Collaborative, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Collaborative, Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Desai, Gyawali, Endpoints used in phase III randomized controlled trials of treatment options for COVID-19, EClinicalMedicine
Dimairo, Pallmann, Wason, Todd, Jaki et al., The Adaptive designs CONSORT Extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design, BMJ
Duvignaud, Lhomme, Onaisi, Sitta, Gelley et al., Coverage Study Group. Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE), Clin Microbiol Infect
Eldridge, Ashby, Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method, Int J Epidemiol
Emmel, Greenhalgh, Manzano, Mmonaghan, Dalkin, Doing Realist Research
Fischer, Eron, Holman, Cohen, Fang et al., Molnupiravir, an oral antiviral treatment for COVID-19, medRxiv
Glasziou, Tikkinen, The RECOVERY trial platform: a milestone in the development and execution of treatment evaluation during an epidemic, J R Soc Med
Glover, Methodology (equal), Resources (equal), Software (equal)
Godfrey, Funding acquisition, Investigation (equal)
Gordon, Juszczak, Montgomery, Howard, Guthrie, The COVID-19 pandemic has highlighted the need to invest in care home research infrastructure, Age Ageing
Griesel, Wagner, Mikolajewska, Stegemann, Fichtner et al., Inhaled corticosteroids for the treatment of COVID-19, Cochrane Database Syst Rev
Guthrie, Investigation (equal), Methodology (equal), Writing -reviewing and editing
Hallett, UK Covid-19 Inquiry. Module 1-The Resilience and Preparedness of the United Kingdom
Halpin, Singh, Hadfield, Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective, Eur Respir J
Heath, Galiza, Baxter, Boffito, Browne et al., nCoV-302 Study Group. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine, N Engl J Med
Hewitt, None
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with COVID-19, N Engl J Med
Horby, Mafham, Linsell, Bell, Staplin et al., Effect of hydroxychloroquine in hospitalized patients with COVID-19, N Engl J Med
Horby, Mafham, Precovery, Group, Casirivimab and imdevimab in patients admitted to hospital with covid-19 (recovery): a randomised, controlled, open-label, platform trial, Lancet
Howard, Investigation (equal), Methodology (equal), Writing -reviewing and editing
Hsu, Lee, Hua, Lai, Tang et al., Inhaled corticosteroid for patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials, J Microbiol Immunol Infect
Humphrey, Dosanjh, Hiemstra, Richter, Chen-Xu et al., PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V), Trials
Investigators, Gordon, Mouncey, Al-Beidh, Rowan et al., Interleukin-6 receptor antagonists in critically ill patients with COVID-19, N Engl J Med
Jaki, Methodology (equal), Resources (equal), Software (equal)
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother
Juszczak, None
Kanani, Waller, Winn, COVID-19 Response Identifying a Clinical Lead for All Care Homes 12 May 2020
Lasserson, None
Leighton, None
Leighton, Sprange, Robinson, Rick, Establishing COVID-19 research in UK care homes -infrastructure challenges for trial design, Eur J Public Health
Leyland, Investigation (equal)
Lim, Brittain, Duley, Edwards, Gordon et al., Blinded randomised controlled trial of low-dose Adjuvant Steroids in Adults admitted to hospital with Pandemic influenza (ASAP): a trial 'in hibernation', ready for rapid activation, Health Technol Assess
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ
Matsuyama, Kawase, Nao, Shirato, Ujike et al., The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, J Virol
Meakin, Project administration (equal), Validation (equal), Visualisation (equal)
Montgomery, None
Mori, Katayama, Nukaga, Triple therapy with hydroxychloroquine, azithromycin, and ciclesonide for COVID-19 pneumonia, J Microbiol Immunol Infect
Ogollah, ), Formal analysis (equal), Methodology (equal), Resources (equal), Software (equal)
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science
Park, Harari, Dron, Lester, Thorlund et al., An overview of platform trials with a checklist for clinical readers, J Clin Epidemiol
Parker, James, Brawley, Clarke, Hoyle et al., Systemic Therapy for Advanced or Metastatic Prostate cancer: Evaluation of Drug Efficacy (STAMPEDE) investigators. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial, Lancet
Passmore, None
Pawson, Evidence Based Policy: A Realist Perspective
Pawson, Middle range theory and programme theory evaluation: from provenance to practice
Pawson, Tilley, Realistic Evaluation
Polack, Thomas, Kitchin, Absalon, Gurtman et al., C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Quinlan, None
Reddy, Jogvanshi, Naikwadi, Sonti, Nirmatrelvir and ritonavir combination: an antiviral therapy for COVID-19, Expert Rev Anti Infect Ther
Rick, None
Robinson, Allen, Darby, Fox, Gordon et al., Contamination in complex healthcare trials: the falls in care homes (FinCH) study experience, BMC Med Res Methodol
Royal, None
Sadoff, Gray, Vandebosch, Cárdenas, Shukarev et al., ENSEMBLE Study Group. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19, N Engl J Med
Sano, Kimizuka, Fujikura, Hisada, Watanabe et al., COVID-19 in older adults: retrospective cohort study in a tertiary hospital in Japan, Geriatr Gerontol Int
Sare, Gray, Bath, Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta analysis, Eur Heart J
Saville, Berry, Efficiencies of platform clinical trials: a vision of the future, Clin Trials
Schaffner, Skoner, Ciclesonide: a safe and effective inhaled corticosteroid for the treatment of asthma, J Asthma Allergy
Shackleton, Essays on Montesquieu and on the Enlightenment
Shen, Investigation (equal), Methodology (equal). Pip Logan, doi:10.3310/MTRS8833
Singh, Weiss, Goodman, Fisk, Kulkarni et al., Niclosamide -a promising treatment for COVID-19, Br J Pharmacol
Smith, PROTECT-V Study Stepped up in Fight against COVID
Susan D Shenkin, None
Terada, Fujita, Kawahara, Hirasawa, Kinoshita et al., Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: an open-label, single-center phase 3 randomized clinical trial, EClinicalMedicine
Terada-Hirashima, Suzuki, Tsujimoto, Hamamoto, Uemura et al., Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: a randomized trial, Drug Discov Ther
Upton, Project administration (equal), Validation (equal), Visualisation (equal)
Vaessen, Leeuw, Mind the Gap: Evaluation and the Disciplines
Van Der Molen, Kocks, The efficacy and safety of inhaled corticosteroids: are we ignoring the potential advantages of ciclesonide?, NPJ Prim Care Respir Med
Vogelmeier, Hering, Lewin, Sander, Bethke, Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care, Respir Med
Voysey, Clemens, Madhi, Weckx, Folegatti et al., Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Whitehead, Sample-size calculations for ordered categorical-data, Stat Med
Yamasaki, Ooka, Tsuchida, Nakamura, Hagiwara et al., The peripheral lymphocyte count as a predictor of severe COVID-19 and the effect of treatment with ciclesonide, Virus Res
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial, medRxiv
Yu, Choi, Ryoo, Cheong, Huh et al., Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: a living review and meta-analysis, PLOS ONE
DOI record:
{
"DOI": "10.3310/mtrs8833",
"ISSN": [
"2046-4924"
],
"URL": "http://dx.doi.org/10.3310/MTRS8833",
"abstract": "<jats:sec id=\"abs1-1\"><jats:title>Background</jats:title>\n<jats:p>Coronavirus disease-2019 was associated with significant mortality and morbidity in care homes in 2020–1. Repurposed antiviral drugs might reduce morbidity and mortality through reducing viral transmission, infection, replication and inflammation. We aimed to compare the safety and efficacy of potential antiviral drugs in care home residents.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-2\"><jats:title>Methods</jats:title>\n<jats:p>We designed a cluster-randomised, open-label, blinded end-point platform trial to test drugs in a postexposure prophylaxis paradigm. Participants aged 65+ years from United Kingdom care homes, with or without nursing, were eligible for participation. Care homes were to be allocated at random by computer to administer 42 days of antiviral agent (ciclesonide or niclosamide) plus standard care versus standard care alone to residents. The primary outcome at 60 days after randomisation comprised the most serious outcome, which was defined as all-cause mortality, all-cause hospitalisation, severe acute respiratory syndrome coronavirus 2 infection or no infection. Analysis would be by intention to treat using ordinal logistic regression. Other outcomes included individual components of the primary outcome, transmission, plus health economic and process evaluation outcomes. The planned sample size was 300 care homes corresponding to 9600 residents. With ~40% of care homes predicted to develop an outbreak during the trial, we needed to recruit 750 homes/24,000 residents.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-3\"><jats:title>Results</jats:title>\n<jats:p>We initiated the trial including protocol, approvals, insurance, website, database, data algorithms, intervention selection and training materials. We built a network of principal investigators and staff (91) and care homes (299) to support the trial. However, we never contracted care homes or general practitioners since the trial was stopped in September 2021, as vaccination in care homes had significantly reduced infections. Multiple delays significantly delayed the start date, such as: (1) reduced prioritisation of pandemic trials in 2021; (2) cumbersome mechanisms for choosing the investigational medicinal products; (3) contracting between National Institute for Health and Care Research and the investigational medicinal product manufacturers; (4) publicising the investigational medicinal products; (5) identification of sufficient numbers of care homes; (6) identification and contracting with several thousand general practitioners; (7) limited research nurse availability and (8) identification of adequate insurance to cover care homes for research. Generic challenges included working across the four home nations with their different structures and regulations.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-4\"><jats:title>Limitations</jats:title>\n<jats:p>The feasibility of contracting between the sponsor and the principal investigators, general practitioners and care homes; screening, consent and treatment of care home residents; data acquisition and the potential benefit of postexposure prophylaxis were never tested.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-5\"><jats:title>Conclusions</jats:title>\n<jats:p>The success of vaccination meant that the role of postexposure prophylaxis of coronavirus disease-2019 in care home residents was not tested. Significant progress was made in developing the infrastructure and expertise necessary for a large-scale clinical trial of investigational medicinal products in United Kingdom care homes.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-6\"><jats:title>Future work</jats:title>\n<jats:p>The role of postexposure prophylaxis of coronavirus disease-2019 in care home residents remains undefined. Significant logistical barriers to conducting research in care homes need to be removed urgently before future studies are possible. Further work is required to develop the infrastructure for clinical trials of investigational medicinal products in care homes. Serious consideration should be given to building and then hibernating a pandemic-ready platform trial suitable for care home research.</jats:p>\n</jats:sec>\n<jats:sec id=\"abs1-7\"><jats:title>Funding</jats:title>\n<jats:p>This article presents independent research funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme as award number NIHR133443.</jats:p>\n</jats:sec>",
"alternative-id": [
"10.3310/MTRS8833"
],
"assertion": [
{
"label": "Free to read",
"name": "free",
"value": "This content has been made freely available to all."
},
{
"group": {
"label": "Article History",
"name": "article_history"
},
"label": "contractual_start_date",
"name": "contractual_start_date",
"value": "01-2021"
},
{
"group": {
"label": "Article History",
"name": "article_history"
},
"label": "editorial review begun",
"name": "editorial_review_begun",
"value": "10-2023"
},
{
"group": {
"label": "Article History",
"name": "article_history"
},
"label": "Accepted for publication",
"name": "accepted_for_publication",
"value": "11-2024"
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0003-2734-5132",
"affiliation": [
{
"name": "Stroke Trials Unit, Mental Health & Clinical Neuroscience, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Bath",
"given": "Philip M",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-5773-8733",
"affiliation": [
{
"name": "Infections, Immunity and Microbes, School of Life Sciences, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Ball",
"given": "Jonathan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2997-5090",
"affiliation": [
{
"name": "Division of Pharmacy Practice and Policy, School of Pharmacy, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Boyd",
"given": "Matthew",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2049-9406",
"affiliation": [
{
"name": "Department of Clinical and Experimental Medicine, Surrey Health Economics Centre, University of Surrey, Guildford, UK"
}
],
"authenticated-orcid": true,
"family": "Gage",
"given": "Heather",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9454-2668",
"affiliation": [
{
"name": "Department of Clinical and Experimental Medicine, Surrey Health Economics Centre, University of Surrey, Guildford, UK"
}
],
"authenticated-orcid": true,
"family": "Glover",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "c/o Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"family": "Godfrey",
"given": "Maureen",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4191-4880",
"affiliation": [
{
"name": "Advanced Care Research Centre, Usher Institute, University of Edinburgh, Edinburgh, UK"
}
],
"authenticated-orcid": true,
"family": "Guthrie",
"given": "Bruce",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7924-1792",
"affiliation": [
{
"name": "Department of Population Medicine, Cardiff University, Cardiff, UK"
}
],
"authenticated-orcid": true,
"family": "Hewitt",
"given": "Jonathan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3071-2338",
"affiliation": [
{
"name": "Division of Psychiatry, University College London, London, UK"
}
],
"authenticated-orcid": true,
"family": "Howard",
"given": "Robert",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1096-188X",
"affiliation": [
{
"name": "MRC Biostatistics Unit, University of Cambridge, Cambridge, UK"
}
],
"authenticated-orcid": true,
"family": "Jaki",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5500-2247",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Juszczak",
"given": "Edmund",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8274-5580",
"affiliation": [
{
"name": "Warwick Medical School, University of Warwick, Coventry, UK"
}
],
"authenticated-orcid": true,
"family": "Lasserson",
"given": "Daniel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5208-0274",
"affiliation": [
{
"name": "Lifespan and Population Sciences, School of Medicine, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Leighton",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bramcote, Nottingham, UK"
}
],
"family": "Leyland",
"given": "Val",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7694-3051",
"affiliation": [
{
"name": "Respiratory Medicine, Nottingham University Hospitals NHS Trust, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Shen Lim",
"given": "Wei",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6657-2381",
"affiliation": [
{
"name": "Unit of Injury, Inflammation and Recovery, School of Medicine, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Logan",
"given": "Pip",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8593-3421",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Meakin",
"given": "Garry",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0450-1606",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Montgomery",
"given": "Alan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5777-4117",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Ogollah",
"given": "Reuben",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2858-5509",
"affiliation": [
{
"name": "Centre for Public Health, Institute for Clinical Sciences, Queen’s University Belfast, Belfast, UK"
}
],
"authenticated-orcid": true,
"family": "Passmore",
"given": "Peter",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3012-6646",
"affiliation": [
{
"name": "Digital Health & Digital Research Service, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Quinlan",
"given": "Philip",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7713-9834",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Rick",
"given": "Caroline",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4560-6036",
"affiliation": [
{
"name": "University of Nottingham Health Service, Cripps Health Centre, University Park, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Royal",
"given": "Simon",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7375-4776",
"affiliation": [
{
"name": "Advanced Care Research Centre, Usher Institute, University of Edinburgh, Edinburgh, UK"
}
],
"authenticated-orcid": true,
"family": "Shenkin",
"given": "Susan D",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2352-6315",
"affiliation": [
{
"name": "Nottingham Clinical Trials Unit, University of Nottingham, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Upton",
"given": "Clare",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1676-9853",
"affiliation": [
{
"name": "NIHR Applied Research Collaboration-East Midlands (ARC-EM), Institute of Mental Health, Nottingham, UK"
}
],
"authenticated-orcid": true,
"family": "Gordon",
"given": "Adam L",
"sequence": "additional"
}
],
"container-title": "Health Technology Assessment",
"container-title-short": "Health Technol Assess",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"journalslibrary.nihr.ac.uk"
]
},
"created": {
"date-parts": [
[
2025,
4,
11
]
],
"date-time": "2025-04-11T16:29:11Z",
"timestamp": 1744388951000
},
"deposited": {
"date-parts": [
[
2025,
4,
11
]
],
"date-time": "2025-04-11T16:29:26Z",
"timestamp": 1744388966000
},
"funder": [
{
"DOI": "10.13039/501100000664",
"award": [
"NIHR133443"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000664",
"id-type": "DOI"
}
],
"name": "Health Technology Assessment programme"
}
],
"indexed": {
"date-parts": [
[
2025,
4,
12
]
],
"date-time": "2025-04-12T04:13:38Z",
"timestamp": 1744431218333,
"version": "3.40.4"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
4
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
1
]
],
"date-time": "2025-04-01T00:00:00Z",
"timestamp": 1743465600000
}
}
],
"link": [
{
"URL": "https://njl-admin.nihr.ac.uk/document/download/2047589",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.journalslibrary.nihr.ac.uk/sites/journalslibrary/files/journal_data/MTRS8833/MTRS8833.xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://njl-admin.nihr.ac.uk/document/download/2047589",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "6221",
"original-title": [],
"page": "1-26",
"prefix": "10.3310",
"published": {
"date-parts": [
[
2025,
4
]
]
},
"published-online": {
"date-parts": [
[
2025,
4
]
]
},
"publisher": "National Institute for Health and Care Research",
"reference": [
{
"article-title": "Prophylactic treatment of COVID-19 in care homes trial (PROTECT-CH)",
"author": "Bath",
"first-page": "2022",
"journal-title": "medRxiv",
"key": "key2025041121515700_ref1-bib1",
"year": "2022"
},
{
"author": "Center for Systems Science and Engineering",
"key": "key2025041121515700_ref2-bib2",
"volume-title": "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)",
"year": "2021"
},
{
"author": "Office of National Statistics",
"key": "key2025041121515700_ref3-bib3",
"volume-title": "Number of Deaths in Care Homes Notified to the Care Quality Commission, England",
"year": "2020"
},
{
"DOI": "10.1093/ageing/afab080",
"article-title": "Impact of COVID-19 on care-home mortality and life expectancy in Scotland",
"author": "Burton",
"doi-asserted-by": "crossref",
"first-page": "1029",
"journal-title": "Age Ageing",
"key": "key2025041121515700_ref4-bib4",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref5-bib5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"article-title": "Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK",
"author": "Voysey",
"doi-asserted-by": "crossref",
"first-page": "99",
"journal-title": "Lancet",
"key": "key2025041121515700_ref6-bib6",
"volume": "397",
"year": "2021"
},
{
"article-title": "Molnupiravir, an oral antiviral treatment for COVID-19",
"author": "Fischer",
"first-page": "2021",
"journal-title": "medRxiv",
"key": "key2025041121515700_ref7-bib7",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref8-bib8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1080/14787210.2023.2241638",
"article-title": "Nirmatrelvir and ritonavir combination: an antiviral therapy for COVID-19",
"author": "Navitha Reddy",
"doi-asserted-by": "crossref",
"first-page": "943",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "key2025041121515700_ref9-bib9",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28889",
"article-title": "Comparative efficacy and safety of nirmatrelvir/ritonavir and molnupiravir for COVID-19: a systematic review and meta-analysis",
"author": "Amani",
"doi-asserted-by": "crossref",
"first-page": "e28889",
"journal-title": "J Med Virol",
"key": "key2025041121515700_ref10-bib10",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref11-bib11",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Lancet",
"key": "key2025041121515700_ref12-bib12",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial",
"author": "Angus",
"doi-asserted-by": "crossref",
"first-page": "1317",
"journal-title": "JAMA",
"key": "key2025041121515700_ref13-bib13",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2022926",
"article-title": "Effect of hydroxychloroquine in hospitalized patients with COVID-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "2030",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref14-bib14",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref15-bib15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "key2025041121515700_ref16-bib16",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with COVID-19",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "1491",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref17-bib17",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Lancet",
"key": "key2025041121515700_ref18-bib18",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2101544",
"article-title": "Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19",
"author": "Sadoff",
"doi-asserted-by": "crossref",
"first-page": "2187",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref19-bib19",
"volume": "384",
"year": "2021"
},
{
"article-title": "Recovery Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with covid-19 (recovery): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"first-page": "665",
"key": "key2025041121515700_ref20-bib20",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "2049",
"journal-title": "Lancet",
"key": "key2025041121515700_ref21-bib21",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107659",
"article-title": "Safety and efficacy of NVX-CoV2373 COVID-19 vaccine",
"author": "Heath",
"doi-asserted-by": "crossref",
"first-page": "1172",
"journal-title": "N Engl J Med",
"key": "key2025041121515700_ref22-bib22",
"volume": "385",
"year": "2021"
},
{
"article-title": "Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial",
"author": "Yu",
"first-page": "2021",
"journal-title": "medRxiv",
"key": "key2025041121515700_ref23-bib23",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"article-title": "Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Recovery Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "605",
"journal-title": "Lancet",
"key": "key2025041121515700_ref24-bib24",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1126/science.abl4784",
"article-title": "An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19",
"author": "Owen",
"doi-asserted-by": "crossref",
"first-page": "1586",
"journal-title": "Science",
"key": "key2025041121515700_ref25-bib25",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2713",
"article-title": "Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "key2025041121515700_ref26-bib26",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1093/ageing/afac052",
"article-title": "The COVID-19 pandemic has highlighted the need to invest in care home research infrastructure",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "afac052",
"journal-title": "Age Ageing",
"key": "key2025041121515700_ref27-bib27",
"volume": "51",
"year": "2022"
},
{
"article-title": "Inhaled corticosteroids for the treatment of COVID-19",
"author": "Griesel",
"journal-title": "Cochrane Database Syst Rev",
"key": "key2025041121515700_ref28-bib28",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1111/bph.15843",
"article-title": "Niclosamide – a promising treatment for COVID-19",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "3250",
"journal-title": "Br J Pharmacol",
"key": "key2025041121515700_ref29-bib29",
"volume": "179",
"year": "2022"
},
{
"DOI": "10.1016/j.eclinm.2020.100403",
"article-title": "Endpoints used in phase III randomized controlled trials of treatment options for COVID-19",
"author": "Desai",
"doi-asserted-by": "crossref",
"first-page": "100403",
"journal-title": "EClinicalMedicine",
"key": "key2025041121515700_ref31-bib31",
"volume": "23",
"year": "2020"
},
{
"DOI": "10.1161/STROKEAHA.108.527044",
"article-title": "Ordinal reanalysis of the SHEP trial",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "e145",
"journal-title": "Stroke",
"key": "key2025041121515700_ref32-bib32",
"volume": "39",
"year": "2008"
},
{
"DOI": "10.1161/STROKEAHA.107.509893",
"article-title": "Use of ordinal outcomes in vascular prevention trials: comparison with binary outcomes in published stroke trials",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "2817",
"journal-title": "Stroke",
"key": "key2025041121515700_ref33-bib33",
"volume": "39",
"year": "2008"
},
{
"DOI": "10.1093/eurheartj/ehn299",
"article-title": "Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta analysis",
"author": "Sare",
"doi-asserted-by": "crossref",
"first-page": "2031",
"journal-title": "Eur Heart J",
"key": "key2025041121515700_ref34-bib34",
"volume": "29",
"year": "2008"
},
{
"DOI": "10.1161/STROKEAHA.106.474080",
"article-title": "Can we improve the statistical analysis of stroke trials? Statistical reanalysis of functional outcomes in stroke trials",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "1911",
"journal-title": "Stroke",
"key": "key2025041121515700_ref35-bib35",
"volume": "38",
"year": "2007"
},
{
"DOI": "10.1136/bmj.e5661",
"article-title": "2010 statement: extension to cluster randomised trials",
"author": "Campbell",
"doi-asserted-by": "crossref",
"first-page": "e5661",
"journal-title": "BMJ",
"key": "key2025041121515700_ref36-bib36",
"volume": "345",
"year": "2012"
},
{
"DOI": "10.1136/bmj.m115",
"article-title": "The Adaptive designs CONSORT Extension (ACE) statement: a checklist with explanation and elaboration guideline for reporting randomised trials that use an adaptive design",
"author": "Dimairo",
"doi-asserted-by": "crossref",
"first-page": "m115",
"journal-title": "BMJ",
"key": "key2025041121515700_ref37-bib37",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1161/STROKEAHA.107.509893",
"article-title": "Optimising the analysis of stroke prevention trials: converting dichotomous vascular outcomes into ordinal measures",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "2817",
"journal-title": "Stroke",
"key": "key2025041121515700_ref38-bib38",
"volume": "39",
"year": "2008"
},
{
"DOI": "10.1161/STROKEAHA.111.641456",
"article-title": "Statistical analysis of the primary outcome in acute stroke trials",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "1171",
"journal-title": "Stroke",
"key": "key2025041121515700_ref39-bib39",
"volume": "43",
"year": "2012"
},
{
"DOI": "10.1002/sim.4780122404",
"article-title": "Sample-size calculations for ordered categorical-data",
"author": "Whitehead",
"doi-asserted-by": "crossref",
"first-page": "2257",
"journal-title": "Stat Med",
"key": "key2025041121515700_ref40-bib40",
"volume": "12",
"year": "1993"
},
{
"DOI": "10.1111/j.1747-4949.2008.00184.x",
"article-title": "Calculation of sample size for stroke trials assessing functional outcome: comparison of binary and ordinal approaches",
"author": "The Optimising Analysis of Stroke Trials (OAST) Collaboration",
"doi-asserted-by": "crossref",
"first-page": "78",
"journal-title": "Int J Stroke",
"key": "key2025041121515700_ref41-bib41",
"volume": "3",
"year": "2008"
},
{
"key": "key2025041121515700_ref42-bib42",
"unstructured": "Competition & Markets Authority. Care homes market study final report. 30 November 2017."
},
{
"DOI": "10.1016/S2666-7568(20)30012-X",
"article-title": "Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK",
"author": "Burton",
"doi-asserted-by": "crossref",
"first-page": "e21",
"journal-title": "Lancet Healthy Longev",
"key": "key2025041121515700_ref43-bib43",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1093/ije/dyl129",
"article-title": "Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method",
"author": "Eldridge",
"doi-asserted-by": "crossref",
"first-page": "1292",
"journal-title": "Int J Epidemiol",
"key": "key2025041121515700_ref44-bib44",
"volume": "35",
"year": "2006"
},
{
"DOI": "10.1002/(SICI)1097-0258(19991230)18:24<3435::AID-SIM365>3.0.CO;2-O",
"article-title": "The role of biostatistics in the prevention, detection and treatment of fraud in clinical trials",
"author": "Buyse",
"doi-asserted-by": "crossref",
"first-page": "3435",
"journal-title": "Stat Med",
"key": "key2025041121515700_ref45-bib45",
"volume": "18",
"year": "1999"
},
{
"DOI": "10.1177/01410768211041245",
"article-title": "The RECOVERY trial platform: a milestone in the development and execution of treatment evaluation during an epidemic",
"author": "Glasziou",
"doi-asserted-by": "crossref",
"first-page": "443",
"journal-title": "J R Soc Med",
"key": "key2025041121515700_ref46-bib46",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1177/1740774515626362",
"article-title": "Efficiencies of platform clinical trials: a vision of the future",
"author": "Saville",
"doi-asserted-by": "crossref",
"first-page": "358",
"journal-title": "Clin Trials",
"key": "key2025041121515700_ref47-bib47",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/j.jclinepi.2020.04.025",
"article-title": "An overview of platform trials with a checklist for clinical readers",
"author": "Park",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Clin Epidemiol",
"key": "key2025041121515700_ref48-bib48",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(18)32486-3",
"article-title": "Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial",
"author": "Parker",
"doi-asserted-by": "crossref",
"first-page": "2353",
"journal-title": "Lancet",
"key": "key2025041121515700_ref49-bib49",
"volume": "392",
"year": "2018"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "key2025041121515700_ref50-bib50",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.1186/s12874-020-00925-z",
"article-title": "Contamination in complex healthcare trials: the falls in care homes (FinCH) study experience",
"author": "Robinson",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "BMC Med Res Methodol",
"key": "key2025041121515700_ref51-bib51",
"volume": "20",
"year": "2020"
},
{
"author": "Shackleton",
"key": "key2025041121515700_ref52-bib52",
"year": "1988"
},
{
"DOI": "10.1093/eurpub/ckac129.751",
"article-title": "Establishing COVID-19 research in UK care homes – infrastructure challenges for trial design",
"author": "Leighton",
"doi-asserted-by": "crossref",
"first-page": "ckac129",
"journal-title": "Eur J Public Health",
"key": "key2025041121515700_ref53-bib53",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1186/s13063-023-07128-z",
"article-title": "PROphylaxis for paTiEnts at risk of COVID-19 infecTion (PROTECT-V)",
"author": "Humphrey",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Trials",
"key": "key2025041121515700_ref54-bib54",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1007/s41999-022-00714-5",
"article-title": "Dietary nitrate supplementation for preventing and reducing the severity of winter infections, including COVID-19, in care homes (BEET-Winter): a randomised placebo-controlled feasibility trial",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "1343",
"journal-title": "Eur Geriatr Med",
"key": "key2025041121515700_ref55-bib55",
"volume": "13",
"year": "2022"
},
{
"author": "Kanani",
"key": "key2025041121515700_ref56-bib56",
"volume-title": "COVID-19 Response Identifying a Clinical Lead for All Care Homes 12 May 2020",
"year": "2020"
},
{
"DOI": "10.12688/f1000research.51270.2",
"article-title": "Nitric oxide for the prevention and treatment of viral, bacterial, protozoal and fungal infections",
"author": "Bath",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "F1000Res",
"key": "key2025041121515700_ref58-bib58",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3310/hta19160",
"article-title": "Blinded randomised controlled trial of low-dose Adjuvant Steroids in Adults admitted to hospital with Pandemic influenza (ASAP): a trial ‘in hibernation’, ready for rapid activation",
"author": "Lim",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Health Technol Assess",
"key": "key2025041121515700_ref59-bib59",
"volume": "19",
"year": "2015"
},
{
"author": "Hallett",
"key": "key2025041121515700_ref60-bib60",
"year": "2024"
},
{
"DOI": "10.1371/journal.pone.0294872",
"article-title": "Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: a living review and meta-analysis",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "e0294872",
"journal-title": "PLOS ONE",
"key": "key2025041121515700_ref61-bib61",
"volume": "18",
"year": "2023"
},
{
"author": "Smith",
"key": "key2025041121515700_ref62-bib62",
"volume-title": "PROTECT-V Study Stepped up in Fight against COVID",
"year": "2023"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819",
"journal-title": "Antimicrob Agents Chemother",
"key": "key2025041121515700_ref63-bib63",
"volume": "64",
"year": "2020"
},
{
"article-title": "Ciclesonide: a safe and effective inhaled corticosteroid for the treatment of asthma",
"author": "Schaffner",
"first-page": "25",
"journal-title": "J Asthma Allergy",
"key": "key2025041121515700_ref64-bib64",
"volume": "2",
"year": "2009"
},
{
"DOI": "10.1016/j.rmed.2010.09.016",
"article-title": "Efficacy and safety of ciclesonide in the treatment of 24,037 asthmatic patients in routine medical care",
"author": "Vogelmeier",
"doi-asserted-by": "crossref",
"first-page": "186",
"journal-title": "Respir Med",
"key": "key2025041121515700_ref65-bib65",
"volume": "105",
"year": "2011"
},
{
"DOI": "10.1038/npjpcrm.2014.13",
"article-title": "The efficacy and safety of inhaled corticosteroids: are we ignoring the potential advantages of ciclesonide?",
"author": "van der Molen",
"doi-asserted-by": "crossref",
"first-page": "14013",
"journal-title": "NPJ Prim Care Respir Med",
"key": "key2025041121515700_ref66-bib66",
"volume": "24",
"year": "2014"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "e01648",
"journal-title": "J Virol",
"key": "key2025041121515700_ref67-bib67",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1016/j.virusres.2020.198089",
"article-title": "The peripheral lymphocyte count as a predictor of severe COVID-19 and the effect of treatment with ciclesonide",
"author": "Yamasaki",
"doi-asserted-by": "crossref",
"first-page": "198089",
"journal-title": "Virus Res",
"key": "key2025041121515700_ref68-bib68",
"volume": "290",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01009-2020",
"article-title": "Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective",
"author": "Halpin",
"doi-asserted-by": "crossref",
"first-page": "2001009",
"journal-title": "Eur Respir J",
"key": "key2025041121515700_ref69-bib69",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.jmii.2020.09.003",
"article-title": "Triple therapy with hydroxychloroquine, azithromycin, and ciclesonide for COVID-19 pneumonia",
"author": "Mori",
"doi-asserted-by": "crossref",
"first-page": "109",
"journal-title": "J Microbiol Immunol Infect",
"key": "key2025041121515700_ref70-bib70",
"volume": "54",
"year": "2021"
},
{
"DOI": "10.1111/ggi.14034",
"article-title": "COVID-19 in older adults: retrospective cohort study in a tertiary hospital in Japan",
"author": "Sano",
"doi-asserted-by": "crossref",
"first-page": "1044",
"journal-title": "Geriatr Gerontol Int",
"key": "key2025041121515700_ref71-bib71",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.5582/ddt.2022.01068",
"article-title": "Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: a randomized trial",
"author": "Terada-Hirashima",
"doi-asserted-by": "crossref",
"first-page": "225",
"journal-title": "Drug Discov Ther",
"key": "key2025041121515700_ref72-bib72",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.02.031",
"article-title": "Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE)",
"author": "Duvignaud",
"doi-asserted-by": "crossref",
"first-page": "1010",
"journal-title": "Clin Microbiol Infect",
"key": "key2025041121515700_ref73-bib73",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2022-064374",
"article-title": "Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19)",
"author": "Brodin",
"doi-asserted-by": "crossref",
"first-page": "e064374",
"journal-title": "BMJ Open",
"key": "key2025041121515700_ref74-bib74",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2022.101484",
"article-title": "Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: an open-label, single-center phase 3 randomized clinical trial",
"author": "Terada",
"doi-asserted-by": "crossref",
"first-page": "101484",
"journal-title": "EClinicalMedicine",
"key": "key2025041121515700_ref75-bib75",
"volume": "49",
"year": "2022"
},
{
"DOI": "10.1016/j.jmii.2023.07.008",
"article-title": "Inhaled corticosteroid for patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials",
"author": "Hsu",
"doi-asserted-by": "crossref",
"first-page": "921",
"journal-title": "J Microbiol Immunol Infect",
"key": "key2025041121515700_ref76-bib76",
"volume": "56",
"year": "2023"
},
{
"article-title": "Broad-spectrum, patient-adaptable inhaled niclosamide-lysozyme particles are efficacious against coronaviruses in lethal murine infection models",
"author": "Brunaugh",
"first-page": "2020",
"journal-title": "bioRxiv",
"key": "key2025041121515700_ref77-bib77",
"year": "2020"
},
{
"DOI": "10.1016/j.lanepe.2021.100084",
"article-title": "A randomized, double-blind, placebo-controlled phase 1 trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19",
"author": "Backer",
"doi-asserted-by": "crossref",
"first-page": "100084",
"journal-title": "Lancet Reg Health Eur",
"key": "key2025041121515700_ref78-bib78",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.amsu.2021.102779",
"article-title": "A randomised controlled trial of effectiveness and safety of niclosamide as add on therapy to the standard of care measures in COVID-19 management",
"author": "Abdulamir",
"doi-asserted-by": "crossref",
"first-page": "102779",
"journal-title": "Ann Med Surg",
"key": "key2025041121515700_ref79-bib79",
"volume": "69",
"year": "2021"
},
{
"author": "Pawson",
"key": "key2025041121515700_ref80-bib80",
"volume-title": "Realistic Evaluation",
"year": "1997"
},
{
"DOI": "10.4135/9781849209120",
"author": "Pawson",
"doi-asserted-by": "crossref",
"key": "key2025041121515700_ref81-bib81",
"volume-title": "Evidence Based Policy: A Realist Perspective",
"year": "2006"
},
{
"author": "Pawson",
"key": "key2025041121515700_ref82-bib82",
"volume-title": "Mind the Gap: Evaluation and the Disciplines",
"year": "2010"
},
{
"DOI": "10.4135/9781526451729",
"author": "Emmel",
"doi-asserted-by": "crossref",
"key": "key2025041121515700_ref83-bib83",
"volume-title": "Doing Realist Research",
"year": "2018"
}
],
"reference-count": 81,
"references-count": 81,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.journalslibrary.nihr.ac.uk/hta/published-articles/MTRS8833"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Lessons from the PROTECT-CH COVID-19 platform trial in care homes",
"type": "journal-article",
"update-policy": "https://doi.org/10.3310/crossmarkpolicy"
}