Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials
et al., BMC Infectious Diseases, doi:10.1186/s12879-021-06829-7, NCT04374526, Nov 2021
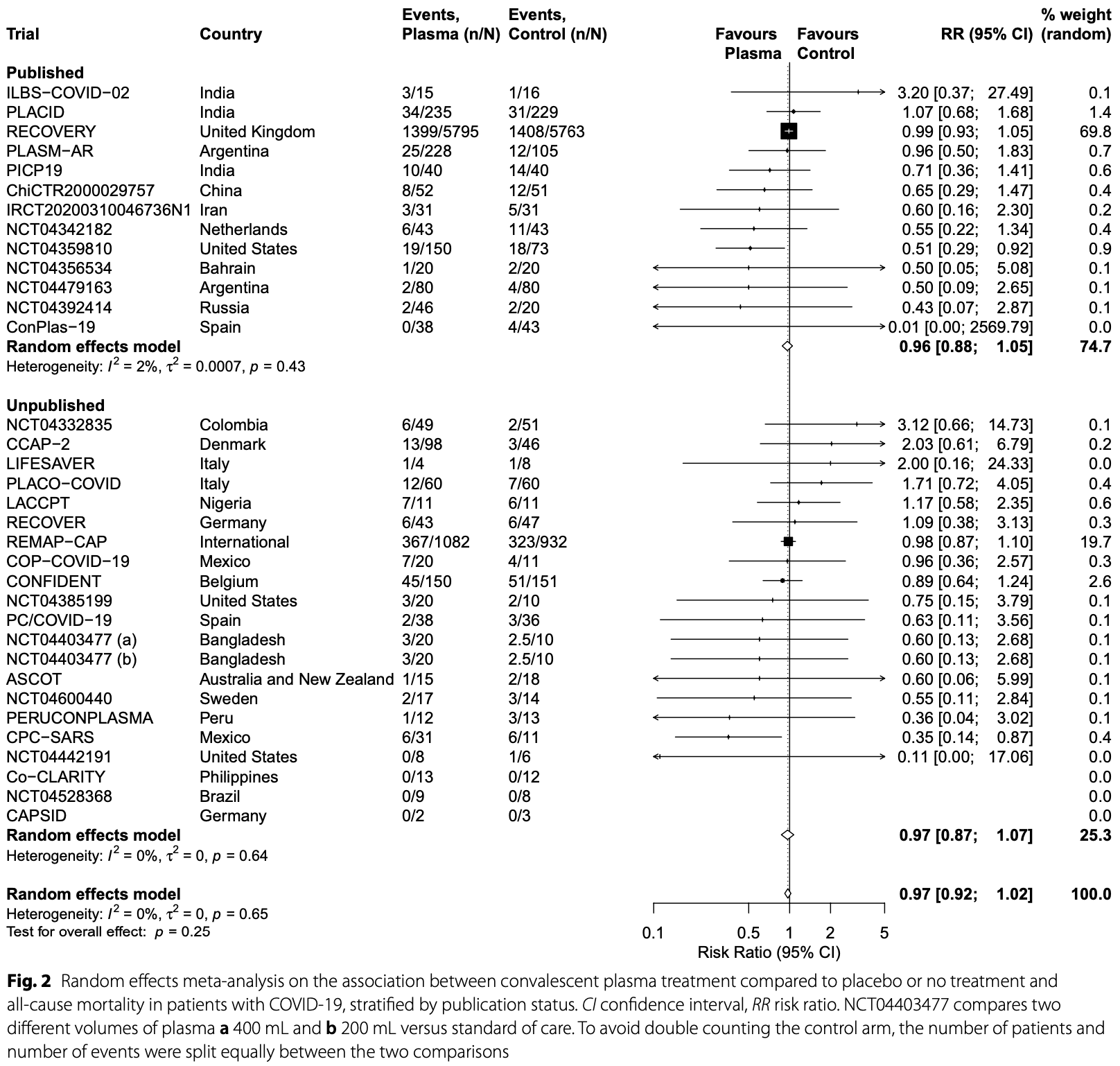
Meta analysis of 33 RCTs with 16,477 patients showing no significant reduction in all-cause mortality with convalescent plasma treatment for COVID-19. There was no difference between subgroups based on patient setting, timing of plasma administration, or antibody titer levels.
Currently there are 58 convalescent plasma studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 3% higher [-2‑7%] |
| Ventilation | 0% higher [-11‑14%] |
| ICU admission | 9% higher [-5‑26%] |
| Hospitalization | 2% higher [-11‑16%] |
| Cases | 40% more [-42‑237%] |
Axfors et al., 20 Nov 2021, Argentina, peer-reviewed, 208 authors, trial NCT04374526 (history).
Contact: lars.hemkens@usb.ch (corresponding author).
Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials
BMC Infectious Diseases, doi:10.1186/s12879-021-06829-7
Background: Convalescent plasma has been widely used to treat COVID-19 and is under investigation in numerous randomized clinical trials, but results are publicly available only for a small number of trials. The objective of this study was to assess the benefits of convalescent plasma treatment compared to placebo or no treatment and all-cause mortality in patients with COVID-19, using data from all available randomized clinical trials, including unpublished and ongoing trials (Open Science Framework, https:// doi. org/ 10. 17605/ OSF. IO/ GEHFX).
Methods: In this collaborative systematic review and meta-analysis, clinical trial registries (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform), the Cochrane COVID-19 register, the LOVE database, and PubMed were searched until April 8, 2021. Investigators of trials registered by March 1, 2021, without published results were contacted via email. Eligible were ongoing, discontinued and completed randomized clinical trials that compared convalescent plasma with placebo or no treatment in COVID-19 patients, regardless of setting or treatment schedule. Aggregated mortality data were extracted from publications or provided by investigators of unpublished trials and combined using the Hartung-Knapp-Sidik-Jonkman random effects model. We investigated the contribution of unpublished trials to the overall evidence. Results: A total of 16,477 patients were included in 33 trials (20 unpublished with 3190 patients, 13 published with 13,287 patients). 32 trials enrolled only hospitalized patients (including 3 with only intensive care unit patients). Risk of bias was low for 29/33 trials. Of 8495 patients who received convalescent plasma, 1997 died (23%), and of 7982 control patients, 1952 died (24%). The combined risk ratio for all-cause mortality was 0.97 (95% confidence interval: 0.92; 1.02) with between-study heterogeneity not beyond chance (I 2 = 0%). The RECOVERY trial had 69.8% and the unpublished evidence 25.3% of the weight in the meta-analysis. Conclusions: Convalescent plasma treatment of patients with COVID-19 did not reduce all-cause mortality. These results provide strong evidence that convalescent plasma treatment for patients with COVID-19 should not be used outside of randomized trials. Evidence synthesis from collaborations among trial investigators can inform both evidence generation and evidence application in patient care.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-021-06829-7. Additional file 1. Search strategy.
Additional file 2. Email invitation. Additional file 3. Amendments to the protocol. Additional file 4. Identified potentially eligible trials not included in the analysis.
Additional file 5. Risk of bias. Additional file 6. Funnel plot.
Additional file 7. Sensitivity analyses: various meta-analytic approaches.
Acknowledgements We would like to express our warm gratitude to all participating patients and convalescent plasma donors. We thank Katja Suter and Sina Ullrich, University of Basel, for their administrative assistance. For their helpful contribution to individual trials, we thank Erica Wood, Iain Gosbell, Richard Charlewood, Thomas Hills, Veronica Hoad, Kristina Kairaitis, Aikaj Jindal, John Gerrard, Hong Foo, Adam Stewart, and Nanette Trask (ASCOT trial); Amalia Bravo-Lindoro, Raúl Carrillo-Esper, Karla Maldonado-Silva, Catalina Casillas-Suárez, Orlando Carrillo-Torres, Sandra Murrieta, Elizabeth Diaz-Padilla, Eli Omar Zavaleta, Yadira Bejar-Ramirez, and Evelyn Cortina-de la Rosa (CPC-SARS trial); Sheri Renaud, Roel Rolando-Almario, and Jacqueline Day (NCT04385199 trial); Sandra Tingsgård, MD, Karen Brorup Heje Pedersen, MD, Michaela Tinggaard, MD, Louise Thorlacius-Ussing, MD, Clara Lundetoft Clausen, MD, Nichlas Hovmand, MD, Simone Bastrup Israelsen, MD, Caecilie Leding,..
References
Abani, Abbas, Abbas, Abbas, Abbasi et al., Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet
Abubakar, Sani, Godman, Kumar, Islam et al., Systematic review on the therapeutic options for COVID-19: clinical evidence of drug efficacy and implications, Infect Drug Resist
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Alqahtani, Abdulrahman, Almadani, Alali, Zamrooni et al., Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, medRxiv
Arabi, Hajeer, Luke, Raviprakash, Balkhy et al., Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia, Emerg Infect Dis
Avendano-Sola, Ramos-Martinez, Munez-Rubio, Ruiz-Antoran, Molina et al., Convalescent plasma for COVID-19: a multicenter, randomized clinical trial, medRxiv
Aviani, Halim, Soeroto, Achmad, Djuwantono, Current views on the potentials of convalescent plasma therapy (CPT) as Coronavirus disease 2019 (COVID-19) treatment: a systematic review and meta-analysis based on recent studies and previous respiratory pandemics, Rev Med Virol, doi:10.1002/rmv.2225
Axfors, Schmitt, Janiaud, Van't Hooft, Abd-Elsalam et al., Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials, Nat Commun
Bajpai, Kumar, Maheshwari, Chhabra, Kale et al., Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial, medRxiv
Bakhtawar, Usman, Khan, Convalescent plasma therapy and its effects on COVID-19 patient outcomes: a systematic review of current literature, Cureus
Baklaushev, Пaвлoвич, Averyanov, Bячecлaвoвич, Sotnikova et al., Safety and efficacy of convalescent plasma for COVID-19: the preliminary results of a clinical trial, J Clin Pract
Bank, None, Centro Médico Naval and FES Iztacala UNAM
Barone, Desimone, Convalescent plasma to treat coronavirus disease 2019 (COVID-19): considerations for clinical trial design, Transfusion
Bin, Centre, Awali, Bahrain. 11 Center for Autoimmune Diseases Research (CREA)
Budhai, Wu, Hall, Strauss, Paradiso et al., How did we rapidly implement a convalescent plasma program?, Transfusion
Chai, Valk, Piechotta, Kimber, Monsef et al., Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review, Cochrane Database Syst Rev
Consultants, None
Ewers, Ioannidis, Plesnila, Access to data from clinical trials in the COVID-19 crisis: open, flexible, and time-sensitive, J Clin Epidemiol
Fabricius, Dandachi, COVID-19 convalescent plasma: from donation to treatment-a systematic review and single center experience, Mo Med
Gharbharan, Jordans, Geurtsvankessel, Hollander, Den et al., Convalescent plasma for COVID-19. A randomized clinical trial, medRxiv
Group, Horby, Estcourt, Peto, Emberson et al., Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, medRxiv
Higgins, Thomas, Chandler, Cumpston, Li et al., Cochrane handbook for systematic reviews of interventions
Higgins, Thompson, Deeks, Altman, Measuring inconsistency in meta-analyses, BMJ
Inthout, Ioannidis, Borm, The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method, BMC Med Res Methodol
Janiaud, Axfors, Saccilotto, Hemkens, COVID-evidence: a living database of trials on interventions for COVID-19
Janiaud, Axfors, Schmitt, Gloy, Ebrahimi et al., Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis, JAMA, doi:10.1001/jama.2021.2747
Juul, Nielsen, Feinberg, Siddiqui, Jørgensen et al., Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project), PLOS Med
Kim, An, Kim, Hwang, Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis, PLoS Med
Klassen, Senefeld, Johnson, Carter, Wiggins et al., The effect of convalescent plasma therapy on COVID-19 patient mortality: systematic review and meta-analysis, Mayo Clinic Proc
Knapp, Hartung, Improved tests for a random effects meta-regression with a single covariate, Stat Med
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19, JAMA
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med
Mair-Jenkins, Saavedra-Campos, Baillie, Cleary, Khaw et al., The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis, J Infect Dis
Meher, Padhy, Das, Mohanty, Agrawal, Effectiveness of convalescent plasma therapy in the treatment of moderate to severe COVID 19 patients: a systematic review and meta-analysis, J Assoc Phys India
Murphy, Estcourt, Grant-Casey, Dzik, International survey of trials of convalescent plasma to treat COVID-19 infection, Transfus Med Rev
O'donnell, Grinsztejn, Cummings, Justman, Lamb et al., A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. medRxiv
Page, Mckenzie, Bossuyt, Boutron, Hoffmann et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews, BMJ
Peng, Rhind, Beckett, Convalescent plasma for the prevention and treatment of COVID-19: a systematic review and quantitative analysis, JMIR Public Health Surveill
Petkova, Antman, Troxel, Pooling data from individual clinical trials in the COVID-19 Era, JAMA
Pouladzadeh, Safdarian, Eshghi, Abolghasemi, Bavani et al., A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm, Intern Emerg Med, doi:10.1007/s11739-021-02734-8
Prasad, Seth, Elavarasi, Efficacy and safety of convalescent plasma for COVID-19: a systematic review and meta-analysis, Indian J Hematol Blood Transfus
Rajendran, Krishnasamy, Rangarajan, Rathinam, Natarajan et al., Convalescent plasma transfusion for the treatment of COVID-19: systematic review, J Med Virol
Rasheed, Fatak, Hashim, Maulood, Kabah et al., The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq, Infez Med
Ray, Paul, Bandopadhyay, 'rozario, Sarif et al., Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial, medRxiv
Rojas, Rodríguez, Monsalve, Acosta-Ampudia, Camacho et al., Convalescent plasma in COVID-19: possible mechanisms of action, Autoimmun Rev
Salholz-Hillel, Grabitz, Pugh-Jones, Strech, Devito, Results availability and timeliness of registered COVID-19 clinical trials: a crosssectional study, medRxiv
Sarkar, Soni, Khanna, Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis, J Med Virol
Schmucker, Blümle, Schell, Schwarzer, Oeller et al., Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research, PLoS ONE
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med
Sterne, Savović, Page, Elbers, Blencowe et al., RoB 2: a revised tool for assessing risk of bias in randomised trials, BMJ
Sterne, Sutton, Ioannidis, Terrin, Jones et al., Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials, BMJ
Talaie, Hosseini, Nazari, Fakhri, Mousavizadeh et al., Is there any potential management against COVID-19? A systematic review and meta-analysis, Daru
Tanne, COVID-19: FDA approves use of convalescent plasma to treat critically ill patients, BMJ
Thorlund, Devereaux, Wetterslev, Guyatt, Ioannidis et al., Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses?, Int J Epidemiol
Trial, RECOVERY trial closes recruitment to convalescent plasma treatment for patients hospitalised with COVID-19 -RECOVERY Trial
Turner, Matthews, Linardatos, Tell, Rosenthal, Selective publication of antidepressant trials and its influence on apparent efficacy, N Engl J Med
Vegivinti, Pederson, Saravu, Gupta, Evanson et al., Efficacy of convalescent plasma therapy for COVID-19: a systematic review and meta-analysis, J Clin Apher, doi:10.1002/jca.21881
Veroniki, Jackson, Viechtbauer, Bender, Bowden et al., Methods to estimate the between-study variance and its uncertainty in meta-analysis, Res Synth Methods
Wang, Huo, Dai, Lv, Yuan et al., Convalescent plasma may be a possible treatment for COVID-19: a systematic review, Int Immunopharmacol
Wang, Wu, Zuo, You, Yang et al., Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis, BMJ Support Palliat Care
Wenjing, Yuanzheng, Li, Tang, Yu, Safety and efficacy of convalescent plasma therapy in severely and critically ill patients with COVID-19: a systematic review with meta-analysis, Aging
Wiksten, Rücker, Schwarzer, Hartung-Knapp method is not always conservative compared with fixed-effect meta-analysis, Stat Med
Zhang, Xi, Pang, Du, Yuan et al., Convalescent plasma in the treatment of severe COVID-19: a systematic review and meta-analysis, Iran J Public Health
Zheng, Liao, Lalu, Tinmouth, Fergusson et al., A scoping review of registered clinical trials of convalescent plasma for COVID-19 and a framework for accelerated synthesis of trial evidence (FAST Evidence), Transfus Med Rev
Zuñiga, Coca, Abeldaño, Villoria, Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis, Ther Adv Respir Dis
DOI record:
{
"DOI": "10.1186/s12879-021-06829-7",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-021-06829-7",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Convalescent plasma has been widely used to treat COVID-19 and is under investigation in numerous randomized clinical trials, but results are publicly available only for a small number of trials. The objective of this study was to assess the benefits of convalescent plasma treatment compared to placebo or no treatment and all-cause mortality in patients with COVID-19, using data from all available randomized clinical trials, including unpublished and ongoing trials (Open Science Framework, <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://doi.org/10.17605/OSF.IO/GEHFX\">https://doi.org/10.17605/OSF.IO/GEHFX</jats:ext-link>).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this collaborative systematic review and meta-analysis, clinical trial registries (ClinicalTrials.gov, WHO International Clinical Trials Registry Platform), the Cochrane COVID-19 register, the LOVE database, and PubMed were searched until April 8, 2021. Investigators of trials registered by March 1, 2021, without published results were contacted via email. Eligible were ongoing, discontinued and completed randomized clinical trials that compared convalescent plasma with placebo or no treatment in COVID-19 patients, regardless of setting or treatment schedule. Aggregated mortality data were extracted from publications or provided by investigators of unpublished trials and combined using the Hartung–Knapp–Sidik–Jonkman random effects model. We investigated the contribution of unpublished trials to the overall evidence.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 16,477 patients were included in 33 trials (20 unpublished with 3190 patients, 13 published with 13,287 patients). 32 trials enrolled only hospitalized patients (including 3 with only intensive care unit patients). Risk of bias was low for 29/33 trials. Of 8495 patients who received convalescent plasma, 1997 died (23%), and of 7982 control patients, 1952 died (24%). The combined risk ratio for all-cause mortality was 0.97 (95% confidence interval: 0.92; 1.02) with between-study heterogeneity not beyond chance (I<jats:sup>2</jats:sup> = 0%). The RECOVERY trial had 69.8% and the unpublished evidence 25.3% of the weight in the meta-analysis.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Convalescent plasma treatment of patients with COVID-19 did not reduce all-cause mortality. These results provide strong evidence that convalescent plasma treatment for patients with COVID-19 should not be used outside of randomized trials. Evidence synthesis from collaborations among trial investigators can inform both evidence generation and evidence application in patient care.</jats:p>\n </jats:sec>",
"alternative-id": [
"6829"
],
"article-number": "1170",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "21 July 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "20 November 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "During the conduct of the study, the following is reported: Dr. Berry reports being employee with ownership role at Berry Consultants (receives payments for statistical modeling and design of REMAP-CAP). Dr. Castillo reports grants from DOST PCHRD. Dr. Daly reports grants from Medical Research Future Fund (Australian Govt) and RBWH. Dr. Denkinger reports grants from German Ministry for Education and Research. Dr. Dumagay reports grants from Philippine Council for Health Research and Development. Dr. Dunachie reports grants from UK Department of Health and Social Care, grants from UK National Institute of Health Research. Dr. Gauiran reports grants from Department of Science and Technology—Philippine Council for Health Research and Development. Dr. Gordon reports grants from NIHR, grants from NIHR Research Professorship (RP-2015-06-18), and non-financial support from NIHR Clinical Research Network. Dr. Higgins reports grants from NHMRC and from the Minderoo Foundation. Dr. Hills reports grants from Health Research Council of New Zealand. Dr. Holm reports grants from Swedish Government Funds for Clinical Research (ALF). Dr. Janssen and Dr. Müller-Tidow report grants from the Federal Ministry of Education and Research in Germany (BMBF) to the RECOVER clinical trial. Dr. Krapp reports grants from Department of Foreign Affairs, Trade, and Development of Canada, grants from Fundación Telefónica del Perú. Dr. J. Lim reports grants from the Department of Science and Technology, Philippine Council for Health Research and Development. Dr. Lucero reports grants from Philippine Council for Health Research and Development. Dr Manrique reports economic support from Grupo ISA Intercolombia for the project development of trial NCT04332835. Drs. McQuilten and Wood report grants from Medical Research Future Fund. Dr. McVerry reports grants from The Pittsburgh Foundation, Translational Breast Cancer Research Consortium, and from UPMC Learning While Doing Program. Mr. Mouncey reports grants from National Institute for Health Research and from the European Union FP7: PREPARE. Dr. Najdovski reports payment from KUL Leuven to Belgian Red Cross for supply of convalescent plasma. Dr. Nichol reports grants from Health Research Board of Ireland. Dr. D. Roberts reports grants from the National Institute for Health (UKRIDHSC COVID-19 Rapid Response Rolling Call—Grant Reference Number COV19-RECPLAS) and the European Commission (SUPPORT-E #101015756). Dr. Rowan reports grants from the European Commission and from the UK National Institute for Health Research. Dr. Shankar-Hari reports grants from National Institute for Health Research UK, grants from UKRI-National Institute for Health Research UK. Dr. Turgeon reports grants from Canadian Institutes of Health Research. Dr. Venkatesh reports grants from Baxter. Dr. Webb reports grants from National Health and Medical Research Council, grants from Minderoo Foundation. Dr. Zacharowski reports grants from EU Horizon 2020. The ASCOT trial team (Drs Bowen, Daly, Davis, Denholm, Hammond, Jha, L. Lim, McQuilten, Molton, Morpeth, O’Sullivan, Paterson, Perry, Price, Rees, Roberts, Rogers, Sasadeusz, Snelling, Tong, Venkatesh, Wood) is funded by grants from from Royal Brisbane and Women’s Hospital Foundation, Pratt Foundation, Minderoo Foundation, BHP Foundation, Hospital Research Foundation, Macquarie Group Foundation, Health Research Council of New Zealand, Australian Partnership for Preparedness Research on Infectious Disease Emergencies (APPRISE), and the collection and supply of convalescent plasma was conducted within Lifeblood’s funding arrangements. The CONFIDENT trial is funded by the Belgian KCE (blood establishments received payment for the convalescent plasma supplied in the clinical trial). REMAP-CAP was supported in part by funding from UKRIDHSC COVID-19 Rapid Response Rolling Call (Grant Reference Number COV19-RECPLAS). Collection of convalescent plasma for REMAP-CAP was funded by the Department of Health and Social Care, UK. The IRCT20200310046736N1 trial was supported by the Ahvaz Jundishapur University of Medical Sciences (Grant No. R.AJUMS.REC.1399.003, Dr. Pouladzadeh). The PC-COVID-19 Group is supported by the Universidad del Rosario, IDCBIS, ISA Group and Suramericana (Colombia). Outside the submitted work, the following is reported: Dr. Axfors reports postdoctoral grants from the Knut and Alice Wallenberg Foundation, Uppsala University, the Swedish Society of Medicine, the Blanceflor Foundation, and the Sweden-America Foundation. Dr. Aomar reports personal fees from SOBI, GEDEON RICHTER, and GSK. Dr. Benfield reports grants from Novo Nordisk Foundation, Simonsen Foundation, Lundbeck Foundation, Kai Hansen Foundation, Erik and Susanna Olesen’s Charitable Fund; grants and personal fees from GSK, Pfizer, Gilead; and personal fees from Boehringer Ingelheim, MSD, and Pentabase ApS. Dr. Estcourt reports being an investigator on the RECOVERY trial. Dr. Gordon reports personal fees from GlaxoSmithKline, Bristol Myers Squibb, and 30 Respiratory. Dr. Jha reports grants and personal fees from Baxter Healthcare, personal fees from Astra Zeneca, grants from NephroPlus. Dr. Laterre reports personal fees from Adrenomed. Dr. McVerry reports grants from NIH/NHLBI and Bayer Pharmaceuticals, Inc. Dr. Mondragon reports financial activities outside the submitted work (employment at Johnson & Johnson). Dr. Perry reports partner being employed at CSL and owning shares in CSL. Dr. Paterson reports involvement with ALLIANCE trial of COVID-19 treatments. Dr. J. Roberts reports other COVID-19 related trials (in different patient groups): tocilizumab in ICU patients; hydroxychloroquine dosing in ICU patients; planned study of remdesivir pharmacokinetics in patients during expanded access program; and in silico evaluation of ivermectin dosing. Dr Sasadeusz reports grants from various Pharma companies including Gilead Sciences, Abvvie, Merck, and Takeda. Dr. Zacharowski reports personal fees from Biotest AG, CSL Behring, GE Heathcare, and is President of the ESAIC."
}
],
"author": [
{
"affiliation": [],
"family": "Axfors",
"given": "Cathrine",
"sequence": "first"
},
{
"affiliation": [],
"family": "Janiaud",
"given": "Perrine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmitt",
"given": "Andreas M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "van’t Hooft",
"given": "Janneke",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Emily R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haber",
"given": "Noah A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abayomi",
"given": "Akin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abduljalil",
"given": "Manal",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdulrahman",
"given": "Abdulkarim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Acosta-Ampudia",
"given": "Yeny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguilar-Guisado",
"given": "Manuela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Al-Beidh",
"given": "Farah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alejandria",
"given": "Marissa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alfonso",
"given": "Rachelle N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ali",
"given": "Mohammad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlQahtani",
"given": "Manaf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "AlZamrooni",
"given": "Alaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anaya",
"given": "Juan-Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ang",
"given": "Mark Angelo C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aomar",
"given": "Ismael F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Argumanis",
"given": "Luis E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Averyanov",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baklaushev",
"given": "Vladimir P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balionis",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benfield",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berry",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Birocco",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonifacio",
"given": "Lynn B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bowen",
"given": "Asha C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bown",
"given": "Abbie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabello-Gutierrez",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camacho",
"given": "Bernardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camacho-Ortiz",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Campbell-Lee",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cao",
"given": "Damon H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cardesa",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carnate",
"given": "Jose M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castillo",
"given": "German Jr. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cavallo",
"given": "Rossana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chowdhury",
"given": "Fazle R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chowdhury",
"given": "Forhad U. H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ciccone",
"given": "Giovannino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cingolani",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Climacosa",
"given": "Fresthel Monica M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Compernolle",
"given": "Veerle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cortez",
"given": "Carlo Francisco N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costa Neto",
"given": "Abel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D’Antico",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daly",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danielle",
"given": "Franca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davis",
"given": "Joshua S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Rosa",
"given": "Francesco Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Denholm",
"given": "Justin T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Denkinger",
"given": "Claudia M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desmecht",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz-Coronado",
"given": "Juan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Díaz Ponce-Medrano",
"given": "Juan A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donneau",
"given": "Anne-Françoise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dumagay",
"given": "Teresita E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dunachie",
"given": "Susanna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dungog",
"given": "Cecile C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erinoso",
"given": "Olufemi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Escasa",
"given": "Ivy Mae S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estcourt",
"given": "Lise J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evans",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Evasan",
"given": "Agnes L. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fareli",
"given": "Christian J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernandez-Sanchez",
"given": "Veronica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galassi",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallo",
"given": "Juan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Patricia J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Patricia L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garcia",
"given": "Jesus A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garigliany",
"given": "Mutien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garza-Gonzalez",
"given": "Elvira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gauiran",
"given": "Deonne Thaddeus V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaviria García",
"given": "Paula A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giron-Gonzalez",
"given": "Jose-Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Almaguer",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gordon",
"given": "Anthony C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gothot",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grass Guaqueta",
"given": "Jeser Santiago",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Green",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grimaldi",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hammond",
"given": "Naomi E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harvala",
"given": "Heli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heralde",
"given": "Francisco M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herrick",
"given": "Jesica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Higgins",
"given": "Alisa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hills",
"given": "Thomas E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hines",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holm",
"given": "Karin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoque",
"given": "Ashraful",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoste",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ignacio",
"given": "Jose M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ivanov",
"given": "Alexander V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Janssen",
"given": "Maike",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jennings",
"given": "Jeffrey H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jha",
"given": "Vivekanand",
"sequence": "additional"
},
{
"affiliation": [],
"family": "King",
"given": "Ruby Anne N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kjeldsen-Kragh",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Klenerman",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kotecha",
"given": "Aditya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krapp",
"given": "Fiorella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labanca",
"given": "Luciana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laing",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landin-Olsson",
"given": "Mona",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laterre",
"given": "Pierre-François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Lyn-Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lim",
"given": "Jodor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ljungquist",
"given": "Oskar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Llaca-Díaz",
"given": "Jorge M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Robles",
"given": "Concepción",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López-Cárdenas",
"given": "Salvador",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lopez-Plaza",
"given": "Ileana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucero",
"given": "Josephine Anne C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lundgren",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Macías",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maganito",
"given": "Sandy C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malundo",
"given": "Anna Flor G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manrique",
"given": "Rubén D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manzini",
"given": "Paola M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcos",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marquez",
"given": "Ignacio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martínez-Marcos",
"given": "Francisco Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mata",
"given": "Ana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McArthur",
"given": "Colin J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McQuilten",
"given": "Zoe K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McVerry",
"given": "Bryan J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Menon",
"given": "David K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyfroidt",
"given": "Geert",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mirasol",
"given": "Ma. Angelina L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Misset",
"given": "Benoît",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molton",
"given": "James S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mondragon",
"given": "Alric V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monsalve",
"given": "Diana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moradi Choghakabodi",
"given": "Parastoo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morpeth",
"given": "Susan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mouncey",
"given": "Paul R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moutschen",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Müller-Tidow",
"given": "Carsten",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murphy",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Najdovski",
"given": "Tome",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nichol",
"given": "Alistair D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Henrik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Novak",
"given": "Richard M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Sullivan",
"given": "Matthew V. N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Olalla",
"given": "Julian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osibogun",
"given": "Akin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Osikomaiya",
"given": "Bodunrin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Oyonarte",
"given": "Salvador",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pardo-Oviedo",
"given": "Juan M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Mahesh C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paterson",
"given": "David L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peña-Perez",
"given": "Carlos A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perez-Calatayud",
"given": "Angel A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez-Alba",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perkina",
"given": "Anastasia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perry",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pouladzadeh",
"given": "Mandana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poyato",
"given": "Inmaculada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Price",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Quero",
"given": "Anne Kristine H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Md. M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Md. S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramesh",
"given": "Mayur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Santana",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rasmussen",
"given": "Magnus",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rees",
"given": "Megan A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rego",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roberts",
"given": "Jason A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roberts",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez",
"given": "Yhojan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez-Baño",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rogers",
"given": "Benjamin A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rojas",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romero",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rowan",
"given": "Kathryn M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saccona",
"given": "Fabio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Safdarian",
"given": "Mehdi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santos",
"given": "Maria Clariza M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sasadeusz",
"given": "Joe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scozzari",
"given": "Gitana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shankar-Hari",
"given": "Manu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sharma",
"given": "Gorav",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Snelling",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soto",
"given": "Alonso",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tagayuna",
"given": "Pedrito Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tang",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tatem",
"given": "Geneva",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teofili",
"given": "Luciana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tong",
"given": "Steven Y. C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turgeon",
"given": "Alexis F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veloso",
"given": "Januario D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Venkatesh",
"given": "Balasubramanian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ventura-Enriquez",
"given": "Yanet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Webb",
"given": "Steve A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiese",
"given": "Lothar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wikén",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wood",
"given": "Erica M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yusubalieva",
"given": "Gaukhar M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zacharowski",
"given": "Kai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zarychanski",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khanna",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moher",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goodman",
"given": "Steven N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ioannidis",
"given": "John P. A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hemkens",
"given": "Lars G.",
"sequence": "additional"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
20
]
],
"date-time": "2021-11-20T11:02:28Z",
"timestamp": 1637406148000
},
"deposited": {
"date-parts": [
[
2021,
11,
20
]
],
"date-time": "2021-11-20T23:03:18Z",
"timestamp": 1637449398000
},
"funder": [
{
"DOI": "10.13039/501100001711",
"award": [
"31CA30_196190"
],
"doi-asserted-by": "crossref",
"name": "swiss national science foundation"
},
{
"DOI": "10.13039/100009827",
"doi-asserted-by": "publisher",
"name": "laura and john arnold foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
8,
9
]
],
"date-time": "2024-08-09T05:54:10Z",
"timestamp": 1723182850078
},
"is-referenced-by-count": 45,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
11,
20
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
20
]
],
"date-time": "2021-11-20T00:00:00Z",
"timestamp": 1637366400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
20
]
],
"date-time": "2021-11-20T00:00:00Z",
"timestamp": 1637366400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-021-06829-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-021-06829-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-021-06829-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
11,
20
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
20
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1111/trf.15843",
"author": "P Barone",
"doi-asserted-by": "crossref",
"first-page": "1123",
"issue": "6",
"journal-title": "Transfusion",
"key": "6829_CR1",
"unstructured": "Barone P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019 (COVID-19): considerations for clinical trial design. Transfusion. 2020;60(6):1123–7.",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.3201/eid2209.151164",
"author": "YM Arabi",
"doi-asserted-by": "crossref",
"first-page": "1554",
"issue": "9",
"journal-title": "Emerg Infect Dis",
"key": "6829_CR2",
"unstructured": "Arabi YM, Hajeer AH, Luke T, Raviprakash K, Balkhy H, Johani S, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–61.",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1093/infdis/jiu396",
"author": "J Mair-Jenkins",
"doi-asserted-by": "crossref",
"first-page": "80",
"issue": "1",
"journal-title": "J Infect Dis",
"key": "6829_CR3",
"unstructured": "Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw F-M, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90.",
"volume": "211",
"year": "2015"
},
{
"DOI": "10.1016/j.autrev.2020.102554",
"author": "M Rojas",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Autoimmun Rev",
"key": "6829_CR4",
"unstructured": "Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in COVID-19: possible mechanisms of action. Autoimmun Rev. 2020;19(7): 102554.",
"volume": "19",
"year": "2020"
},
{
"key": "6829_CR5",
"unstructured": "Klassen SA, Senefeld JW, Johnson PW, Carter RE, Wiggins CC, Shoham S, et al. The effect of convalescent plasma therapy on COVID-19 patient mortality: systematic review and meta-analysis. Mayo Clinic Proc. 2021. https://www.mayoclinicproceedings.org/article/S0025-6196(21)00140-3/abstract. Accessed 27 Apr 2021."
},
{
"author": "JH Tanne",
"issue": "368",
"journal-title": "BMJ",
"key": "6829_CR6",
"unstructured": "Tanne JH. COVID-19: FDA approves use of convalescent plasma to treat critically ill patients. BMJ. 2020;26(368): m1256.",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1111/trf.15910",
"author": "A Budhai",
"doi-asserted-by": "crossref",
"first-page": "1348",
"issue": "7",
"journal-title": "Transfusion",
"key": "6829_CR7",
"unstructured": "Budhai A, Wu AA, Hall L, Strauss D, Paradiso S, Alberigo J, et al. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;60(7):1348–55.",
"volume": "60",
"year": "2020"
},
{
"key": "6829_CR8",
"unstructured": "Commissioner O of the FDA issues emergency use authorization for convalescent plasma as potential promising COVID-19 treatment, another achievement in administration’s fight against pandemic. FDA. FDA; 2020. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment. Accessed 14 Apr 2021."
},
{
"key": "6829_CR9",
"unstructured": "Commissioner O of the FDA issues emergency use authorization for convalescent plasma as potential promising COVID-19 treatment, another achievement in administration’s fight against pandemic. FDA. FDA; 2020. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-convalescent-plasma-potential-promising-covid-19-treatment. Accessed 27 Jan 2021."
},
{
"key": "6829_CR10",
"unstructured": "European Commission. An EU programme of COVID-19 convalescent plasma collection and transfusion: Guidance on collection, testing, processing, storage, distribution and monitored use. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf. Accessed 27 Apr 2021."
},
{
"key": "6829_CR11",
"unstructured": "European Commission. COVID-19 convalescent plasma collection. European Commission—European Commission. https://ec.europa.eu/commission/presscorner/detail/en/ip_21_50. Accessed 27 Apr 2021."
},
{
"key": "6829_CR12",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2020;0(0):null."
},
{
"author": "A Agarwal",
"journal-title": "BMJ",
"key": "6829_CR13",
"unstructured": "Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371: m3939.",
"volume": "371",
"year": "2020"
},
{
"author": "L Li",
"first-page": "1",
"issue": "5",
"journal-title": "JAMA",
"key": "6829_CR14",
"unstructured": "Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19. JAMA. 2020;324(5):1–11.",
"volume": "324",
"year": "2020"
},
{
"key": "6829_CR15",
"unstructured": "Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;0(0):null."
},
{
"DOI": "10.1101/2020.11.02.20224303",
"doi-asserted-by": "crossref",
"key": "6829_CR16",
"unstructured": "AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Zamrooni AMA, Hejab AH, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. medRxiv. 2020;2020.11.02.20224303."
},
{
"DOI": "10.1101/2020.07.01.20139857",
"doi-asserted-by": "crossref",
"key": "6829_CR17",
"unstructured": "Gharbharan A, Jordans CCE, Geurtsvankessel C, Hollander JG den, Karim F, Mollema FPN, et al. Convalescent plasma for COVID-19. A randomized clinical trial. medRxiv. 2020;2020.07.01.20139857."
},
{
"DOI": "10.1101/2020.10.25.20219337",
"doi-asserted-by": "crossref",
"key": "6829_CR18",
"unstructured": "Bajpai M, Kumar S, Maheshwari A, Chhabra K, Kale P, Gupta A, et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. medRxiv. 2020;2020.10.25.20219337."
},
{
"key": "6829_CR19",
"unstructured": "Avendano-Sola C, Ramos-Martinez A, Munez-Rubio E, Ruiz-Antoran B, Molina RM de, Torres F, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. medRxiv. 2020;2020.08.26.20182444."
},
{
"DOI": "10.1101/2020.11.25.20237883",
"doi-asserted-by": "crossref",
"key": "6829_CR20",
"unstructured": "Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Lahiri A, et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. medRxiv. 2020;2020.11.25.20237883."
},
{
"key": "6829_CR21",
"unstructured": "RECOVERY Trial. RECOVERY trial closes recruitment to convalescent plasma treatment for patients hospitalised with COVID-19 — RECOVERY Trial. https://www.recoverytrial.net/news/statement-from-the-recovery-trial-chief-investigators-15-january-2021-recovery-trial-closes-recruitment-to-convalescent-plasma-treatment-for-patients-hospitalised-with-covid-19. Accessed 27 Jan 2021."
},
{
"key": "6829_CR22",
"unstructured": "Janiaud P, Axfors C, Saccilotto R, Hemkens L. COVID-evidence: a living database of trials on interventions for COVID-19. 2020. https://osf.io/gehfx/. Accessed 17 May 2021."
},
{
"author": "MJ Page",
"journal-title": "BMJ",
"key": "6829_CR23",
"unstructured": "Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.",
"volume": "372",
"year": "2021"
},
{
"key": "6829_CR24",
"unstructured": "Living overview of the evidence (L·OVE). https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d?utm=epdb_en. Accessed 17 Dec 2020."
},
{
"author": "JAC Sterne",
"journal-title": "BMJ",
"key": "6829_CR25",
"unstructured": "Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.",
"volume": "366",
"year": "2019"
},
{
"author": "JAC Sterne",
"journal-title": "BMJ",
"key": "6829_CR26",
"unstructured": "Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343: d4002.",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1186/1471-2288-14-25",
"author": "J IntHout",
"doi-asserted-by": "crossref",
"first-page": "25",
"issue": "14",
"journal-title": "BMC Med Res Methodol",
"key": "6829_CR27",
"unstructured": "IntHout J, Ioannidis JPA, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian–Laird method. BMC Med Res Methodol. 2014;18(14):25.",
"volume": "18",
"year": "2014"
},
{
"key": "6829_CR28",
"unstructured": "Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions. www.training.cochrane.org/handbook. Accessed 14 Apr 2021."
},
{
"DOI": "10.1136/bmj.327.7414.557",
"author": "JPT Higgins",
"doi-asserted-by": "crossref",
"first-page": "557",
"issue": "7414",
"journal-title": "BMJ",
"key": "6829_CR29",
"unstructured": "Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.",
"volume": "327",
"year": "2003"
},
{
"DOI": "10.1002/jrsm.1164",
"author": "AA Veroniki",
"doi-asserted-by": "crossref",
"first-page": "55",
"issue": "1",
"journal-title": "Res Synth Methods",
"key": "6829_CR30",
"unstructured": "Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79.",
"volume": "7",
"year": "2016"
},
{
"key": "6829_CR31",
"unstructured": "Food and Drug Administration. Convalescent plasma EUA letter of authorization, March 9, 2021. https://www.fda.gov/media/141477/download."
},
{
"DOI": "10.1016/j.tmrv.2020.06.003",
"author": "M Murphy",
"doi-asserted-by": "crossref",
"first-page": "151",
"issue": "3",
"journal-title": "Transfus Med Rev",
"key": "6829_CR32",
"unstructured": "Murphy M, Estcourt L, Grant-Casey J, Dzik S. International survey of trials of convalescent plasma to treat COVID-19 infection. Transfus Med Rev. 2020;34(3):151–7.",
"volume": "34",
"year": "2020"
},
{
"key": "6829_CR33",
"unstructured": "World Bank Country and Lending Groups—World Bank Data Help. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed 14 Apr 2021."
},
{
"key": "6829_CR34",
"unstructured": "Commissioner O of the. FDA In Brief: FDA updates emergency use authorization for COVID-19 convalescent plasma to reflect new data. FDA. 2021. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-updates-emergency-use-authorization-covid-19-convalescent-plasma-reflect-new-data. Accessed 14 Apr 2021."
},
{
"DOI": "10.1002/sim.1482",
"author": "G Knapp",
"doi-asserted-by": "crossref",
"first-page": "2693",
"issue": "17",
"journal-title": "Stat Med",
"key": "6829_CR35",
"unstructured": "Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–710.",
"volume": "22",
"year": "2003"
},
{
"DOI": "10.1002/sim.6879",
"author": "A Wiksten",
"doi-asserted-by": "crossref",
"first-page": "2503",
"issue": "15",
"journal-title": "Stat Med",
"key": "6829_CR36",
"unstructured": "Wiksten A, Rücker G, Schwarzer G. Hartung–Knapp method is not always conservative compared with fixed-effect meta-analysis. Stat Med. 2016;35(15):2503–15.",
"volume": "35",
"year": "2016"
},
{
"key": "6829_CR37",
"unstructured": "COMET-Initiative. Core outcome set developers’ response to COVID-19 (7th July 2020). http://www.comet-initiative.org/Studies/Details/1538. Accessed 7 Oct 2020."
},
{
"key": "6829_CR38",
"unstructured": "Group TRC, Horby PW, Estcourt L, Peto L, Emberson JR, Staplin N, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. medRxiv. 2021;2021.03.09.21252736."
},
{
"key": "6829_CR39",
"unstructured": "O’Donnell M, Grinsztejn B, Cummings M, Justman J, Lamb M, Eckhardt C, et al. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. medRxiv. 2021. http://www.epistemonikos.org/documents/7d0ffefb14ca100bd77eb742f59c27fa693fa217."
},
{
"author": "VP Baklaushev",
"first-page": "38",
"issue": "2",
"journal-title": "J Clin Pract",
"key": "6829_CR40",
"unstructured": "Baklaushev VP, Пaвлoвич БB, Averyanov AV, Bячecлaвoвич AA, Sotnikova AG, Гeннaдиeвнa CA, et al. Safety and efficacy of convalescent plasma for COVID-19: the preliminary results of a clinical trial. J Clin Pract. 2020;11(2):38–50.",
"volume": "11",
"year": "2020"
},
{
"key": "6829_CR41",
"unstructured": "Abani O, Abbas A, Abbas F, Abbas M, Abbasi S, Abbass H, et al. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00897-7/abstract. Accessed 21 May 2021."
},
{
"DOI": "10.1007/s11739-021-02734-8",
"author": "M Pouladzadeh",
"doi-asserted-by": "publisher",
"journal-title": "Intern Emerg Med",
"key": "6829_CR42",
"unstructured": "Pouladzadeh M, Safdarian M, Eshghi P, Abolghasemi H, Bavani AG, Sheibani B, et al. A randomized clinical trial evaluating the immunomodulatory effect of convalescent plasma on COVID-19-related cytokine storm. Intern Emerg Med. 2021. https://doi.org/10.1007/s11739-021-02734-8.",
"year": "2021"
},
{
"DOI": "10.1093/ije/dyn179",
"author": "K Thorlund",
"doi-asserted-by": "crossref",
"first-page": "276",
"issue": "1",
"journal-title": "Int J Epidemiol",
"key": "6829_CR43",
"unstructured": "Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JPA, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38(1):276–86.",
"volume": "38",
"year": "2009"
},
{
"DOI": "10.1016/j.jclinepi.2020.10.008",
"author": "M Ewers",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "J Clin Epidemiol",
"key": "6829_CR44",
"unstructured": "Ewers M, Ioannidis JPA, Plesnila N. Access to data from clinical trials in the COVID-19 crisis: open, flexible, and time-sensitive. J Clin Epidemiol. 2021;130:143–6.",
"volume": "130",
"year": "2021"
},
{
"DOI": "10.1016/j.tmrv.2020.06.005",
"author": "K Zheng",
"doi-asserted-by": "crossref",
"first-page": "158",
"issue": "3",
"journal-title": "Transfus Med Rev",
"key": "6829_CR45",
"unstructured": "Zheng K, Liao G, Lalu MM, Tinmouth A, Fergusson DA, Allan DS. A scoping review of registered clinical trials of convalescent plasma for COVID-19 and a framework for accelerated synthesis of trial evidence (FAST Evidence). Transfus Med Rev. 2020;34(3):158–64.",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1371/journal.pmed.1003293",
"author": "S Juul",
"doi-asserted-by": "crossref",
"issue": "9",
"journal-title": "PLOS Med",
"key": "6829_CR46",
"unstructured": "Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLOS Med. 2020;17(9): e1003293.",
"volume": "17",
"year": "2020"
},
{
"author": "KL Chai",
"journal-title": "Cochrane Database Syst Rev",
"key": "6829_CR47",
"unstructured": "Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;10: CD013600.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-22446-z",
"author": "C Axfors",
"doi-asserted-by": "crossref",
"first-page": "2349",
"issue": "1",
"journal-title": "Nat Commun",
"key": "6829_CR48",
"unstructured": "Axfors C, Schmitt AM, Janiaud P, van’t Hooft J, Abd-Elsalam S, Abdo EF, et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials. Nat Commun. 2021;12(1):2349.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.13042",
"author": "E Petkova",
"doi-asserted-by": "crossref",
"first-page": "543",
"issue": "6",
"journal-title": "JAMA",
"key": "6829_CR49",
"unstructured": "Petkova E, Antman EM, Troxel AB. Pooling data from individual clinical trials in the COVID-19 Era. JAMA. 2020;324(6):543.",
"volume": "324",
"year": "2020"
},
{
"key": "6829_CR50",
"unstructured": "Continuous monitoring of pooled international trials of convalescent plasma for COVID-19 hospitalized patients. NYU Langone Health. https://med.nyu.edu/departments-institutes/population-health/divisions-sections-centers/biostatistics/research/continuous-monitoring-pooled-international-trials-convalescent-plasma-covid19-hospitalized-patients. Accessed 22 Apr 2021."
},
{
"author": "RA Abeldaño Zuñiga",
"journal-title": "Ther Adv Respir Dis",
"key": "6829_CR51",
"unstructured": "Abeldaño Zuñiga RA, Coca SM, Abeldaño GF, González-Villoria RAM. Clinical effectiveness of drugs in hospitalized patients with COVID-19: a systematic review and meta-analysis. Ther Adv Respir Dis. 2021;15: 17534666211007214.",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.2147/IDR.S289037",
"author": "AR Abubakar",
"doi-asserted-by": "crossref",
"first-page": "4673",
"journal-title": "Infect Drug Resist",
"key": "6829_CR52",
"unstructured": "Abubakar AR, Sani IH, Godman B, Kumar S, Islam S, Jahan I, et al. Systematic review on the therapeutic options for COVID-19: clinical evidence of drug efficacy and implications. Infect Drug Resist. 2020;13:4673–95.",
"volume": "13",
"year": "2020"
},
{
"author": "N Bakhtawar",
"issue": "8",
"journal-title": "Cureus.",
"key": "6829_CR53",
"unstructured": "Bakhtawar N, Usman M, Khan MMU. Convalescent plasma therapy and its effects on COVID-19 patient outcomes: a systematic review of current literature. Cureus. 2020;12(8): e9535.",
"volume": "12",
"year": "2020"
},
{
"author": "MM Fabricius",
"first-page": "74",
"issue": "1",
"journal-title": "Mo Med",
"key": "6829_CR54",
"unstructured": "Fabricius MM, Dandachi D. COVID-19 convalescent plasma: from donation to treatment—a systematic review and single center experience. Mo Med. 2021;118(1):74–80.",
"volume": "118",
"year": "2021"
},
{
"author": "MS Kim",
"issue": "12",
"journal-title": "PLoS Med",
"key": "6829_CR55",
"unstructured": "Kim MS, An MH, Kim WJ, Hwang T-H. Comparative efficacy and safety of pharmacological interventions for the treatment of COVID-19: a systematic review and network meta-analysis. PLoS Med. 2020;17(12): e1003501.",
"volume": "17",
"year": "2020"
},
{
"author": "BR Meher",
"first-page": "35",
"issue": "12",
"journal-title": "J Assoc Phys India",
"key": "6829_CR56",
"unstructured": "Meher BR, Padhy BM, Das S, Mohanty RR, Agrawal K. Effectiveness of convalescent plasma therapy in the treatment of moderate to severe COVID 19 patients: a systematic review and meta-analysis. J Assoc Phys India. 2020;68(12):35–43.",
"volume": "68",
"year": "2020"
},
{
"author": "HT Peng",
"issue": "4",
"journal-title": "JMIR Public Health Surveill",
"key": "6829_CR57",
"unstructured": "Peng HT, Rhind SG, Beckett A. Convalescent plasma for the prevention and treatment of COVID-19: a systematic review and quantitative analysis. JMIR Public Health Surveill. 2021;7(4): e25500.",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1002/jmv.25961",
"author": "K Rajendran",
"doi-asserted-by": "crossref",
"first-page": "1475",
"issue": "9",
"journal-title": "J Med Virol",
"key": "6829_CR58",
"unstructured": "Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID-19: systematic review. J Med Virol. 2020;92(9):1475–83.",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26408",
"author": "S Sarkar",
"doi-asserted-by": "crossref",
"first-page": "1111",
"issue": "2",
"journal-title": "J Med Virol",
"key": "6829_CR59",
"unstructured": "Sarkar S, Soni KD, Khanna P. Convalescent plasma is a clutch at straws in COVID-19 management! A systematic review and meta-analysis. J Med Virol. 2021;93(2):1111–8.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s40199-020-00367-4",
"author": "H Talaie",
"doi-asserted-by": "crossref",
"first-page": "765",
"issue": "2",
"journal-title": "Daru",
"key": "6829_CR60",
"unstructured": "Talaie H, Hosseini SM, Nazari M, Fakhri Y, Mousavizadeh A, Vatanpour H, et al. Is there any potential management against COVID-19? A systematic review and meta-analysis. Daru. 2020;28(2):765–77.",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1136/bmjspcare-2020-002554",
"author": "M Wang",
"doi-asserted-by": "crossref",
"first-page": "45",
"issue": "1",
"journal-title": "BMJ Support Palliat Care",
"key": "6829_CR61",
"unstructured": "Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L, et al. Evaluation of current medical approaches for COVID-19: a systematic review and meta-analysis. BMJ Support Palliat Care. 2021;11(1):45–52.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.18632/aging.202195",
"author": "L Wenjing",
"doi-asserted-by": "crossref",
"first-page": "1498",
"issue": "1",
"journal-title": "Aging (Albany NY)",
"key": "6829_CR62",
"unstructured": "Wenjing L, Yuanzheng F, Li J-Y, Tang LV, Yu H. Safety and efficacy of convalescent plasma therapy in severely and critically ill patients with COVID-19: a systematic review with meta-analysis. Aging (Albany NY). 2020;13(1):1498–509.",
"volume": "13",
"year": "2020"
},
{
"author": "X Zhang",
"first-page": "2022",
"issue": "11",
"journal-title": "Iran J Public Health",
"key": "6829_CR63",
"unstructured": "Zhang X, Xi L, Pang F, Du Y, Yuan Q, Shi M, et al. Convalescent plasma in the treatment of severe COVID-19: a systematic review and meta-analysis. Iran J Public Health. 2020;49(11):2022–31.",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.2747",
"author": "P Janiaud",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "6829_CR64",
"unstructured": "Janiaud P, Axfors C, Schmitt AM, Gloy V, Ebrahimi F, Hepprich M, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021. https://doi.org/10.1001/jama.2021.2747.",
"year": "2021"
},
{
"author": "M Prasad",
"first-page": "1",
"journal-title": "Indian J Hematol Blood Transfus",
"key": "6829_CR65",
"unstructured": "Prasad M, Seth T, Elavarasi A. Efficacy and safety of convalescent plasma for COVID-19: a systematic review and meta-analysis. Indian J Hematol Blood Transfus. 2021;16:1–19.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2225",
"author": "JK Aviani",
"doi-asserted-by": "publisher",
"journal-title": "Rev Med Virol",
"key": "6829_CR66",
"unstructured": "Aviani JK, Halim D, Soeroto AY, Achmad TH, Djuwantono T. Current views on the potentials of convalescent plasma therapy (CPT) as Coronavirus disease 2019 (COVID-19) treatment: a systematic review and meta-analysis based on recent studies and previous respiratory pandemics. Rev Med Virol. 2021. https://doi.org/10.1002/rmv.2225.",
"year": "2021"
},
{
"DOI": "10.1002/jca.21881",
"author": "CTR Vegivinti",
"doi-asserted-by": "publisher",
"journal-title": "J Clin Apher",
"key": "6829_CR67",
"unstructured": "Vegivinti CTR, Pederson JM, Saravu K, Gupta N, Evanson KW, Kamrowski S, et al. Efficacy of convalescent plasma therapy for COVID-19: a systematic review and meta-analysis. J Clin Apher. 2021. https://doi.org/10.1002/jca.21881.",
"year": "2021"
},
{
"author": "Y Wang",
"journal-title": "Int Immunopharmacol",
"key": "6829_CR68",
"unstructured": "Wang Y, Huo P, Dai R, Lv X, Yuan S, Zhang Y, et al. Convalescent plasma may be a possible treatment for COVID-19: a systematic review. Int Immunopharmacol. 2021;91: 107262.",
"volume": "91",
"year": "2021"
},
{
"author": "AM Rasheed",
"first-page": "357",
"issue": "3",
"journal-title": "Infez Med",
"key": "6829_CR69",
"unstructured": "Rasheed AM, Fatak DF, Hashim HA, Maulood MF, Kabah KK, Almusawi YA, et al. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez Med. 2020;28(3):357–66.",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1056/NEJMsa065779",
"author": "EH Turner",
"doi-asserted-by": "crossref",
"first-page": "252",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "6829_CR70",
"unstructured": "Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–60.",
"volume": "358",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0176210",
"author": "CM Schmucker",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "PLoS ONE",
"key": "6829_CR71",
"unstructured": "Schmucker CM, Blümle A, Schell LK, Schwarzer G, Oeller P, Cabrera L, et al. Systematic review finds that study data not published in full text articles have unclear impact on meta-analyses results in medical research. PLoS ONE. 2017;12(4): e0176210.",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1101/2021.04.07.21255071",
"doi-asserted-by": "crossref",
"key": "6829_CR72",
"unstructured": "Salholz-Hillel M, Grabitz P, Pugh-Jones M, Strech D, DeVito NJ. Results availability and timeliness of registered COVID-19 clinical trials: a cross-sectional study. medRxiv. 2021;2021.04.07.21255071."
}
],
"reference-count": 72,
"references-count": 72,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-021-06829-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "21"
}