Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial
et al., medRxiv, doi:10.1101/2021.02.22.21252228, Feb 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Tiny early-terminated 34 patient RCT for outpatient treatment showing faster recovery with treatment (not statistically significant). All patients recovered (3 control patients recovered after crossover to the treatment arm) - as per protocol mid-recovery results have priority. There was no mortality and only one hospitalization on day 0 before treatment. There were no severe adverse events.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of not reaching lowest symptom score at day 7 mid-recovery, 60.0% lower, RR 0.40, p = 0.13, treatment 3 of 15 (20.0%), control 6 of 12 (50.0%), NNT 3.3.
|
|
risk of not reaching lowest symptom score at day 5 mid-recovery, 50.0% lower, RR 0.50, p = 0.13, treatment 5 of 15 (33.3%), control 8 of 12 (66.7%), NNT 3.0.
|
|
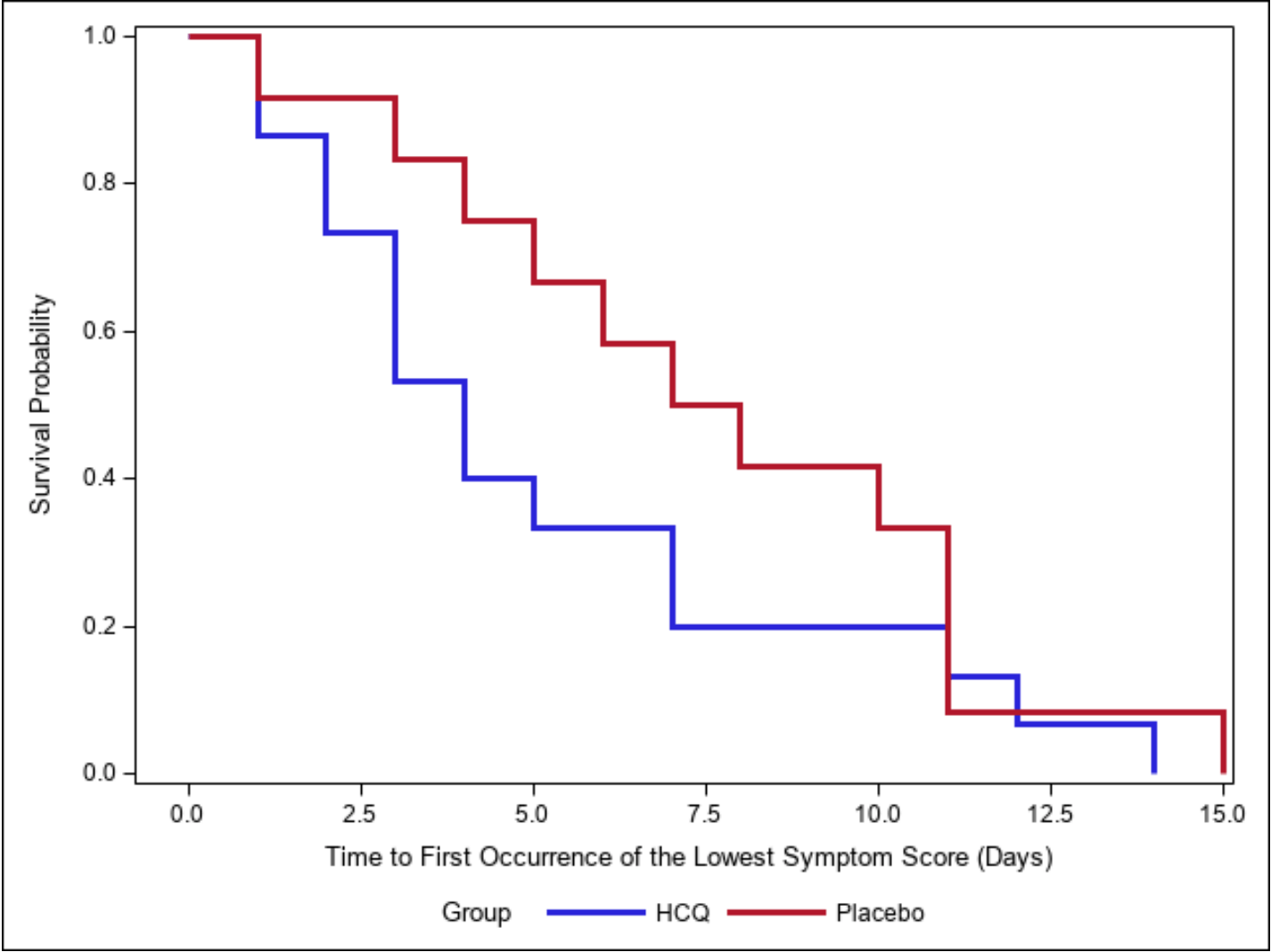
relative time to first occurrence of lowest symptom score, 42.9% lower, relative time 0.57, p = 0.38, treatment median 4.0 IQR 13.0 n=15, control median 7.0 IQR 10.0 n=12.
|
|
relative time to release from quarantine, 27.3% lower, relative time 0.73, p = 0.46, treatment median 8.0 IQR 15.0 n=16, control median 11.0 IQR 14.0 n=13, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Amaravadi et al., 26 Feb 2021, Double Blind Randomized Controlled Trial, USA, preprint, 20 authors, study period 15 April, 2020 - 14 July, 2020, dosage 400mg bid days 1-14.
Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial
doi:10.1101/2021.02.22.21252228
Background Older patients are at risk of increased morbidity and mortality from COVID-19 disease due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). There are few effective treatments for outpatients with COVID-19.
Objective To evaluate the efficacy of hydroxychloroquine to reduce time in quarantine for symptomatic ≥40 years-old COVID-19 patients.
Design A randomized, double-blind, placebo-controlled clinical trial.
Setting Outpatients with polymerase chain reaction confirmed COVID-19 at a University of Pennsylvania affiliated testing center between April 15, 2020 and, July 14, 2020.
Participants Out of 5511 SARS-CoV-2 positive patients, 1072 met initial eligibility criteria for telephonebased recruitment, but only 34 subjects were able to be randomized.
Interventions Hydroxychloroquine 400 mg per twice daily (n=17) or matching placebo (n=17), taken orally for up to 14 days.
Measurements The primary outcome was the time to release from quarantine. Secondary outcomes included the participant-reported secondary infection of co-inhabitants, hospitalization, treatment-related adverse events, time to symptom improvement, and incidence of cardiac arrhythmia.
Results .
References
Alexander, Debono, Mammen, Iorio, Aryal et al., COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine, J Clin Epidemiol, doi:10.1016/j.jclinepi.2020.04.016
Burton, Fort, Seoane, Hospitalization and Mortality among Black Patients and White Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMsa2011686
Group, Horby, Mafham, Linsell, Bell et al., Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2022926
Hwang, Shih, Cani, Group sequential designs using a family of type I error probability spending functions, Stat Med, doi:10.1002/sim.4780091207
Liu, Cao, Xu, Wang, Zhang et al., None
Mehra, Desai, Ruschitzka, Patel, RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis, Lancet, doi:10.1016/S0140-6736(20)31180-6
Northwell, Barnaby, Becker, Chelico, Cohen et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Nurchis, Pascucci, Sapienza, Villani, 'ambrosio et al., Impact of the Burden of COVID-19 in Italy: Results of Disability-Adjusted Life Years (DALYs) and Productivity Loss, Int J Environ Res Public Health, doi:10.3390/ijerph17124233
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., the
Skipper, Pastick, Engen, Bangdiwala, Abassi et al., Hydroxychloroquine in Nonhospitalized Adults With Early COVID-19: A Randomized Trial, Ann Intern Med, doi:10.7326/M20-4207
DOI record:
{
"DOI": "10.1101/2021.02.22.21252228",
"URL": "http://dx.doi.org/10.1101/2021.02.22.21252228",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Older patients are at risk of increased morbidity and mortality from COVID-19 disease due to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). There are few effective treatments for outpatients with COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the efficacy of hydroxychloroquine to reduce time in quarantine for symptomatic ≥40 years-old COVID-19 patients.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>A randomized, double-blind, placebo-controlled clinical trial.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Outpatients with polymerase chain reaction confirmed COVID-19 at a University of Pennsylvania affiliated testing center between April 15, 2020 and, July 14, 2020.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Out of 5511 SARS-CoV-2 positive patients, 1072 met initial eligibility criteria for telephone-based recruitment, but only 34 subjects were able to be randomized.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Hydroxychloroquine 400 mg per twice daily (n=17) or matching placebo (n=17), taken orally for up to 14 days.</jats:p></jats:sec><jats:sec><jats:title>Measurements</jats:title><jats:p>The primary outcome was the time to release from quarantine. Secondary outcomes included the participant-reported secondary infection of co-inhabitants, hospitalization, treatment-related adverse events, time to symptom improvement, and incidence of cardiac arrhythmia.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The median time to release from quarantine for HCQ-treated vs. placebo-treated participants was 8 days (range 4-19 days) vs. 11 days (4-18 days); z-score +0.58, p=n.s. This did not meet the pre-specified criteria for early termination, however, this study was terminated early due to lack of feasibility. There was no mortality in either study arm.</jats:p></jats:sec><jats:sec><jats:title>Limitation</jats:title><jats:p>Since this study was terminated early due to a lack of feasibility, no conclusion can be made about the efficacy of hydroxychloroquine as a treatment for COVID-19 patients 40 years of age or older quarantined at home.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The design of this remotely conducted study could guide testing of other more promising agents during the COVID-19 pandemic.</jats:p></jats:sec><jats:sec><jats:title>Trial registration</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"http://Clinicaltrials.gov\">Clinicaltrials.gov</jats:ext-link> identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04329923\">NCT04329923</jats:ext-link></jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
2,
26
]
]
},
"author": [
{
"affiliation": [],
"family": "Amaravadi",
"given": "Ravi K.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Giles",
"given": "Lydia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carberry",
"given": "Mary",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4050-6925",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hyman",
"given": "Matthew C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frank",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nasta",
"given": "Sunita D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walsh",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wileyto",
"given": "E. Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gimotty",
"given": "Phyllis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milone",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teng",
"given": "Edith M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vyas",
"given": "Niraj J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balian",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kolansky",
"given": "Jonathan A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdulhay",
"given": "Nabil M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McGovern",
"given": "Shaun K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gamblin",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doran",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Callahan",
"given": "Paul L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abella",
"given": "Benjamin S.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
2,
26
]
],
"date-time": "2021-02-26T13:40:14Z",
"timestamp": 1614346814000
},
"deposited": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T17:25:21Z",
"timestamp": 1614446721000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
1,
27
]
],
"date-time": "2023-01-27T16:34:54Z",
"timestamp": 1674837294505
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 2,
"issued": {
"date-parts": [
[
2021,
2,
26
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.02.22.21252228",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
2,
26
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
2,
26
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.1"
},
{
"DOI": "10.1056/NEJMsa2011686",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.2"
},
{
"DOI": "10.3390/ijerph17124233",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.3"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.4"
},
{
"DOI": "10.1002/sim.4780091207",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.5"
},
{
"DOI": "10.7326/M20-4207",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.6"
},
{
"DOI": "10.1016/j.jclinepi.2020.04.016",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.7"
},
{
"DOI": "10.1016/S0140-6736(20)31180-6",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.8"
},
{
"DOI": "10.1056/NEJMoa2022926",
"doi-asserted-by": "publisher",
"key": "2021022709250539000_2021.02.22.21252228v1.9"
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.02.22.21252228"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Hydroxychloroquine for SARS-CoV-2 positive patients quarantined at home: The first interim analysis of a remotely conducted randomized clinical trial",
"type": "posted-content"
}
