Association of Funding and Conclusions in Randomized Drug Trials
et al., JAMA, doi:10.1001/jama.290.7.921, Aug 2003
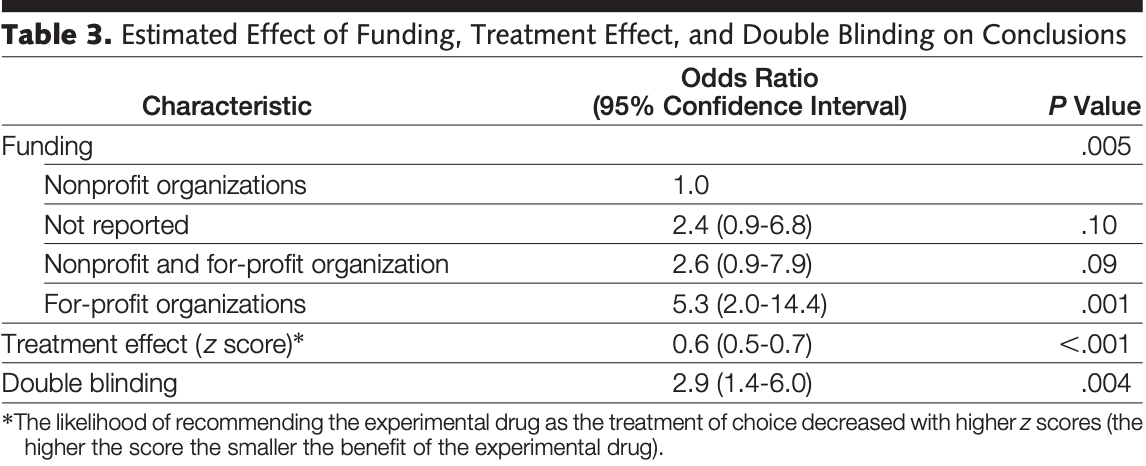
Analysis of randomized drug trials included in Cochrane reviews, showing that trials funded by for-profit organizations were significantly more likely to recommend the experimental drug as treatment of choice (OR 5.3 [2.0-14.4]) compared with trials funded by nonprofit organizations.
Als-Nielsen et al., 20 Aug 2003, peer-reviewed, 4 authors.
Contact: bodil.a@ctu.rh.dk.
Association of Funding and Conclusions in Randomized Drug Trials A Reflection of Treatment Effect or Adverse Events?
Context Previous studies indicate that industry-sponsored trials tend to draw proindustry conclusions. Objective To explore whether the association between funding and conclusions in randomized drug trials reflects treatment effects or adverse events. Design Observational study of 370 randomized drug trials included in metaanalyses from Cochrane reviews selected from the Cochrane Library, May 2001. From a random sample of 167 Cochrane reviews, 25 contained eligible meta-analyses (assessed a binary outcome; pooled at least 5 full-paper trials of which at least 1 reported adequate and 1 reported inadequate allocation concealment). The primary binary outcome from each meta-analysis was considered the primary outcome for all trials included in each meta-analysis. The association between funding and conclusions was analyzed by logistic regression with adjustment for treatment effect, adverse events, and additional confounding factors (methodological quality, control intervention, sample size, publication year, and place of publication). Main Outcome Measure Conclusions in trials, classified into whether the experimental drug was recommended as the treatment of choice or not.
Results The experimental drug was recommended as treatment of choice in 16% of trials funded by nonprofit organizations, 30% of trials not reporting funding, 35% of trials funded by both nonprofit and for-profit organizations, and 51% of trials funded by for-profit organizations (PϽ.001; 2 test). Logistic regression analyses indicated that funding, treatment effect, and double blinding were the only significant predictors of conclusions. Adjusted analyses showed that trials funded by for-profit organizations were significantly more likely to recommend the experimental drug as treatment of choice (odds ratio, 5.3; 95% confidence interval, 2.0-14.4) compared with trials funded by nonprofit organizations. This association did not appear to reflect treatment effect or adverse events. Conclusions Conclusions in trials funded by for-profit organizations may be more positive due to biased interpretation of trial results. Readers should carefully evaluate whether conclusions in randomized trials are supported by data.
References
Adams, Mccall, Gray, Orza, Chalmers, Economic analysis in randomized control trials, Med Care
Alderson, Schierhout, Roberts, Bunn, Colloids versus crystalloids for fluid resuscitation in critically ill patients [Cochrane Review on CD-ROM, Update Software
Altman, Practical Statistics for Medical Research
Balk, Boris, Moskowitz, Correlation of quality measures with estimates of treatment effect in meta-analyses of randomized controlled trials, JAMA
Bekelman, Li, Gross, Scope and impact of financial conflicts of interest in biomedical research: a systematic review, JAMA
Brocklehurst, Hannah, Mcdonald, Interventions for treating bacterial vaginosis in pregnancy
Candelise, Ciccone, Gangliosides for acute ischaemic stroke [Cochrane Review on CD-ROM, Update Software
Clarke, Oxman, Cochrane Reviewers' Handbook 4.1.6
Clifford, Barrowman, Moher, Funding source, trial outcome and reporting quality: are they related? results of a pilot study, BMC Health Serv Res
Colditz, Miller, Mosteller, How study design affects outcomes in comparisons of therapy, I: medical, Stat Med
Couchoud, Cytomegalovirus prophylaxis with antiviral agents for solid organ transplantation [Cochrane Review on CD-ROM, Update Software
Davidoff, Deangelis, Drazen, Sponsorship, authorship, and accountability, JAMA
Davidson, Source of funding and outcome of clinical trials, J Gen Intern Med
Davies, Olson, Gibson, Methotrexate as a steroid sparing agent for asthma in adults [Cochrane Review on CD-ROM
Daya, Gunby, Recombinant versus urinary follicle stimulating hormone for ovarian stimulation in assisted reproduction cycles
Djulbegovic, Lacevic, Cantor, The uncertainty principle and industry-sponsored research, Lancet
Duncan, Jorgensen, Wase, Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice, Stroke
Easterbrook, Berlin, Gopalan, Matthews, Publication bias in clinical research, Lancet
Egger, Davey, Schneider, Minder, Bias in meta-analysis detected by a simple, graphical test, BMJ
Egger, Ju ¨ni, Bartlett, Holenstein, How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? empirical study, Health Technol Assess
Feigin, Rinkel, Algra, Vermeulen, Van Gijn, Calcium antagonists for aneurysmal subarachnoid haemorrhage [Cochrane Review on CD-ROM
Freemantle, Interpreting the results of secondary end points and subgroup analyses in clinical trials: should we lock the crazy aunt in the attic?, BMJ
French, Smaill, Antibiotic regimens for endometritis after delivery [Cochrane Review on CD-ROM, Update Software
Friedberg, Saffran, Stinson, Bennett, Evaluation of conflict of interest in economic analyses of new drugs used in oncology, JAMA
Garner, Gu, Prevention versus treatment for malaria in pregnant women [Cochrane Review on CD-ROM
Gibbs, Harvey, Sterling, Stark, Local treatments for cutaneous warts [Cochrane Review on CD-ROM, Update Software
Gilbert, Mcpeek, Mosteller, Statistics and ethics in surgery and anesthesia, Science
Gluud, Kjaergard, Quality of randomized clincial trials in portal hypertension and other fields of hepatology
Gøtzche, Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal antiinflammatory drugs in rheumatoid arthritis, Control Clin Trials
Gøtzsche, Liberati, Torri, Rossetti, Beware of surrogate outcome measures, Int J Technol Assess Health Care
Henry, Moxey, Carless, Antifibrinolytic use for minimising perioperative allogeneic blood transfusion [Cochrane Review on CD-ROM, Update Software
Horton, Medical editors trial amnesty, Lancet
Hosmer, Lemeshow, Applied Logistic Regression
Huwiler-Mu ¨ntener, Ju ¨ni, Junker, Egger, Quality of reporting of randomized trials as a measure of methodologic quality, JAMA
Ioannou, Doust, Rockey, Terlipressin for acute esophageal variceal hemorrhage [Cochrane Review on CD-ROM
Jacobs, Association between competing interests and conclusions, BMJ
Jadad, Cook, Jones, Methodology and reports of systematic reviews and metaanalyses: a comparison of Cochrane reviews with articles published in paper-based journals, JAMA
Jadad, Moher, Browman, Systematic reviews and meta-analyses on treatment of asthma: critical evaluation, BMJ
Kellner, Ohlsson, Gadomski, Wang, Bronchodilators for bronchiolitis [Cochrane Review on CD-ROM
Kjaergard, Als-Nielsen, Association between competing interests and authors' conclusions: epidemiological study of randomised clinical trials published in the BMJ
Kjaergard, Als-Nielsen, Association between competing interests and authors' conclusions: epidemiological study of randomised clinical trials published in, BMJ
Kjaergard, Nikolova, Gluud, Randomized clinical trials in HEPATOLOGY: predictors of quality, Hepatology
Kjaergard, Villumsen, Gluud, Reported methodological quality and discrepancies between large and small randomized trials in meta-analyses, Ann Intern Med
Lexchin, Bero, Djulbegovic, Clark, Pharmaceutical industry sponsorship and research outcome and quality: systematic review, BMJ
Lima, Moncrieff, Drugs versus placebo for dysthymia [Cochrane Review on CD-ROM, Update Software
Melander, Ahlqvist-Rastad, Meijer, Beermann, Evidence b(i)ased medicine-selective reporting from studies sponsored by pharmaceutical industry: review of studies in new drug applications, BMJ
Moher, Schulz, Altman, Group, The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials, JAMA
Olliaro, Mussano, Amodiaquine for treating malaria [Cochrane Review on CD-ROM, Update Software
Pl, Jarvis, Kitchener, Lilford, Progestagens for endometrial cancer [Cochrane Review on CD-ROM
Pocock, Hughes, Lee, Statistical problems in the reporting of clinical trials: a survey of three medical journals, N Engl J Med
Roberts, Counsell, Assessment of clinical out-comes in acute stroke trials, Stroke
Rochon, Gurwitz, Simms, A study of manufacturer-supported trials of nonsteroidal antiinflammatory drugs in the treatment of arthritis, Arch Intern Med
Roos, Rinkel, Vermeulen, Algra, Van Gijn, Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage [Cochrane Review on CD-ROM, Update Software
Rossetti, Marchetti, Orzalesi, Scorpiglione, Liberati, Is proper methodology associated with the use of a clinically relevant outcome measure? the case of randomized clinical trials on medical treatment of open-angle glaucoma, Online J Curr Clin Trials
Rowe, Spooner, Ducharme, Bretzlaff, Bota, Corticosteroids for preventing relapse following acute exacerbations of asthma [Cochrane Review on CD-ROM, Update Software
Rowe, Spooner, Ducharme, Bretzlaff, Bota, Early emergency department treatment of acute asthma with systemic corticosteroids [Cochrane Review on CD-ROM, Update Software
Schulz, Chalmers, Hayes, Altman, Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials, JAMA
Silagy, Mant, Fowler, Lancaster, Nicotine replacement therapy for smoking cessation [Cochrane Review on CD-ROM, Update Software
Simes, Publication bias: the case for an international registry of clinical trials, J Clin Oncol
Soo, Moayyedi, Deeks, Delaney, Innes et al., Pharmacological interventions for nonulcer dyspepsia [Cochrane Review on CD-ROM
Streiner, Norman, Health Measurement Scales: A Practical Guide to Their Development and Use
Suarez-Almazor, Spooner, Belseck, Shea, Auranofin versus placebo in rheumatoid arthritis [Cochrane Review on CD-ROM, Update Software
Tan, Hannah, Prostaglandins versus oxy-tocin for prelabour rupture of membranes at term
Thompson, Understanding financial conflicts of interest, N Engl J Med
Tinnion, Hanlon, Acellular vaccines for preventing whooping cough in children [Cochrane Review on CD-ROM
Walters, Gibson, Oral corticosteroids for acute exacerbations of chronic obstructive pulmonary disease [Cochrane Review on CD-ROM, Update Software
Yaphe, Edman, Knishkowy, Herman, The association between funding by commercial interests and study outcome in randomized controlled drug trials, Fam Pract
Yusuf, Wittes, Probstfield, Tyroler, Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials, JAMA
Zhang, Schmidt, Do we measure the right end points? a systematic review of primary outcomes in recent neonatal randomized clinical trials, J Pediatr
DOI record:
{
"DOI": "10.1001/jama.290.7.921",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.290.7.921",
"author": [
{
"affiliation": [],
"family": "Als-Nielsen",
"given": "Bodil",
"sequence": "first"
},
{
"affiliation": [],
"family": "Chen",
"given": "Wendong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gluud",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kjaergard",
"given": "Lise L.",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2003,
8,
19
]
],
"date-time": "2003-08-19T20:46:03Z",
"timestamp": 1061325963000
},
"deposited": {
"date-parts": [
[
2016,
12,
15
]
],
"date-time": "2016-12-15T17:29:32Z",
"timestamp": 1481822972000
},
"indexed": {
"date-parts": [
[
2024,
2,
19
]
],
"date-time": "2024-02-19T09:42:03Z",
"timestamp": 1708335723814
},
"is-referenced-by-count": 523,
"issue": "7",
"issued": {
"date-parts": [
[
2003,
8,
20
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2003,
8,
20
]
]
}
},
"language": "en",
"link": [
{
"URL": "http://jamanetwork.com/journals/jama/fullarticle/197132",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "921",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2003,
8,
20
]
]
},
"published-print": {
"date-parts": [
[
2003,
8,
20
]
]
},
"publisher": "American Medical Association (AMA)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.290.7.921"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Reflection of Treatment Effect or Adverse Events?"
],
"title": "Association of Funding and Conclusions in Randomized Drug Trials",
"type": "journal-article",
"volume": "290"
}