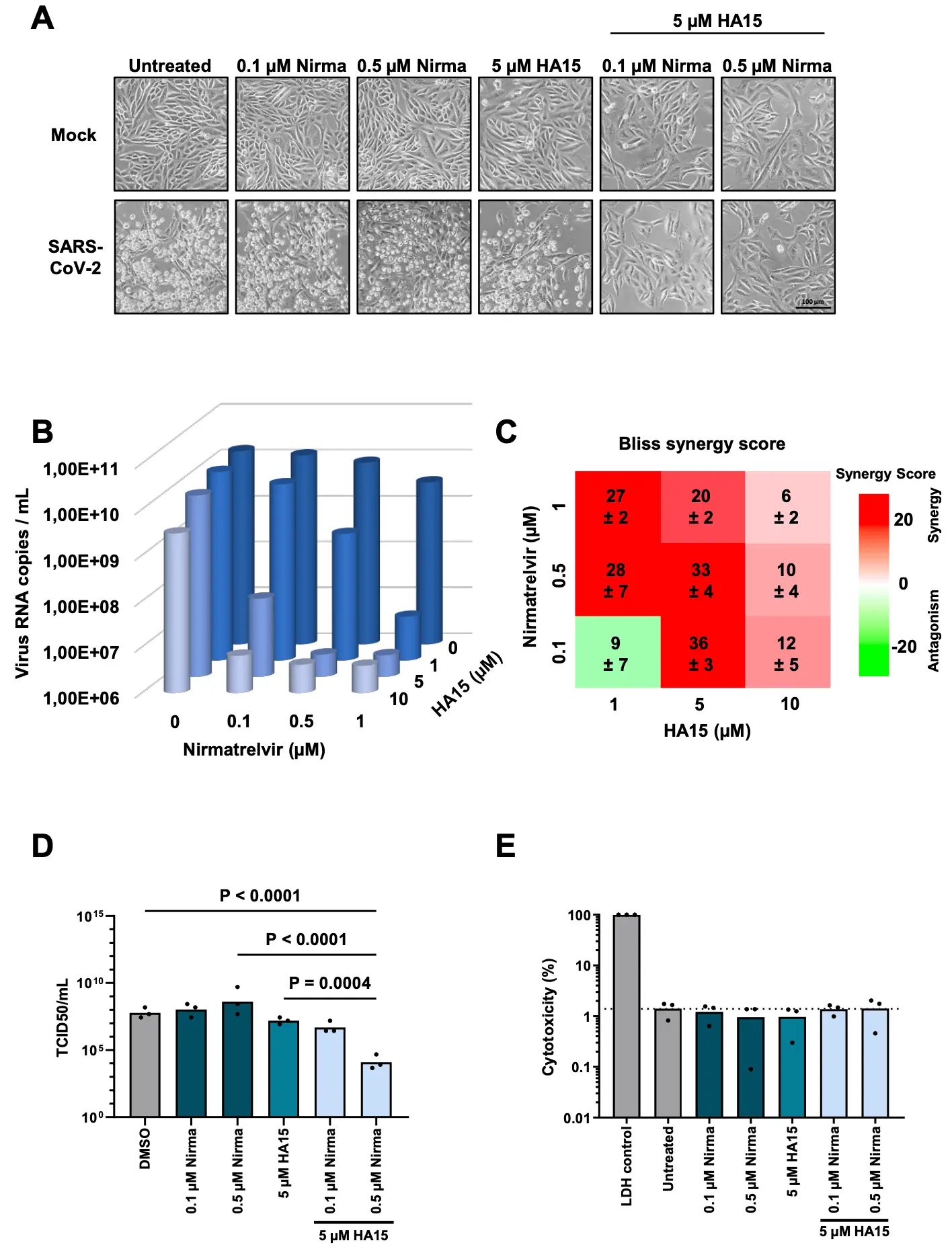
The protease inhibitor Nirmatrelvir synergizes with inhibitors of GRP78 to suppress SARS-CoV-2 replication
et al., bioRxiv, doi:10.1101/2025.03.09.642200, Mar 2025
In vitro and animal study showing strong synergistic effects when combining nirmatrelvir with GRP78 inhibitors.
Al Krad et al., 11 Mar 2025, Germany, preprint, 10 authors.
Contact: mdobbel@unigoettingen.de.
The protease inhibitor Nirmatrelvir synergizes with inhibitors of GRP78 to suppress SARS-CoV-2 replication
doi:10.1101/2025.03.09.642200
Nirmatrelvir, the active compound of the drug Paxlovid, inhibits the Main protease of SARS-CoV-2 (M Pro , 3CL Pro , NSP5). Its therapeutic application reduces but does not abolish the progression of COVID-19 in humans. Here we report a strong synergy of Nirmatrelvir with inhibitors of the ER chaperone GRP78 (HSPA5, BiP). Combining Nirmatrelvir with the GRP78-antagonizing drug candidate HA15 strongly inhibits the replication of SARS-CoV-2, to a far greater extent than either drug alone, as observed by diminished cytopathic effect, levels of detectable virus RNA, TCID 50 titers, accumulation of the non-structural protein 3 (NSP3), as well as Spike and N proteins. The original SARS-CoV-2 strain as well as an Omicron variant were similarly susceptible towards the drug combination. Other GRP78 inhibitors or siRNAs targeting GRP78 also fortified the antiviral effect of Nirmatrelvir. In a hamster model of COVID-19, the combination of Nirmatrelvir with HA15 alleviated pneumonia-induced pulmonary atelectasis more effectively than the single drugs. In conclusion, inhibition of the virus Main protease and cellular GRP78 cooperatively diminishes virus replication and may improve COVID-19 therapy. .
LEGENDS TO SUPPLEMENTAL FIGURESs
References
Abraham, Nohria, Neilan, Asnani, Saji et al., Cardiovascular Drug Interactions With Nirmatrelvir/Ritonavir in Patients With COVID-19: JACC Review Topic of the Week, J Am Coll Cardiol
Amani, Amani, Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: A rapid review and meta-analysis, J Med Virol
Angelini, Akhlaghpour, Neuman, Buchmeier, Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce doublemembrane vesicles, mBio
Blaurock, Breithaupt, Weber, Wylezich, Keller et al., Compellingly high SARS-CoV-2 susceptibility of Golden Syrian hamsters suggests multiple zoonotic infections of pet hamsters during the COVID-19 pandemic, Sci Rep
Carlos, Ha, Yeh, Van Krieken, Tseng et al., The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection, Journal of Biological Chemistry
Casas, GRP78 at the Centre of the Stage in Cancer and Neuroprotection, Frontiers in Neuroscience
Chan, Yuan, Chu, Sridhar, Yuen, COVID-19 drug discovery and treatment options, Nat Rev Microbiol
Corman, Landt, Kaiser, Molenkamp, Meijer et al., Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR, Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin
Dai, Zhang, Jiang, Su, Li et al., Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease, Science
Dobbelstein, Moll, Targeting tumour-supportive cellular machineries in anticancer drug development, Nat Rev Drug Discov
Ha, Shin, Liu, Doche, Lau et al., Targeting stress induction of GRP78 by cardiac glycoside oleandrin dually suppresses cancer and COVID-19, Cell & bioscience
Han, Lv, Moser, Zhou, Woehrle et al., ACE2-independent SARS-CoV-2 virus entry through cell surface GRP78 on monocytes -evidence from a translational clinical and experimental approach, EBioMedicine
Han, Lü, Moser, Woehrle, Zhou et al., Cell surface GRP78 functions as an alternative virus entry receptor on monocytes during SARS-CoV-2 infection, European Respiratory Journal
Hetz, Zhang, Kaufman, Mechanisms, regulation and functions of the unfolded protein response, Nat Rev Mol Cell Biol
Ibrahim, Abdelmalek, Elfiky, GRP78: A cell's response to stress, Life Sci
Jiao, Fan, Ma, Lin, Zhao et al., SARS-CoV-2 nonstructural protein 6 triggers endoplasmic reticulum stress-induced autophagy to degrade STING1, Autophagy
Khongwichit, Sornjai, Jitobaom, Greenwood, Greenwood et al., A functional interaction between GRP78 and Zika virus E protein, Sci Rep
Kreft, Jerman, Lasic, Hevir-Kene, Rizner et al., The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function, Eur J Pharm Sci
Kärber, Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche, Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie
Lee, Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential, Nat Rev Cancer
Li, Hilgenfeld, Whitley, De Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov
Loos, Beijnen, Schinkel, The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism?, Int J Mol Sci
Luo, Fan, Zhang, Ngo, Zhao et al., Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice
Ma, Hendershot, The role of the unfolded protein response in tumour development: friend or foe?, Nat Rev Cancer
Nain, Mukherjee, Karmakar, Paton, Paton et al., GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells, J Virol
Ni, Zhang, Lee, Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting, Biochemical Journal
Owen, Allerton, Anderson, Aschenbrenner, Avery et al., An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science
Pene, Hernandez, Vauloup-Fellous, Garaud-Aunis, Rosenberg, Sequential processing of hepatitis C virus core protein by host cell signal peptidase and signal peptide peptidase: a reassessment, J Viral Hepat
Prikis, Cameron, Paxlovid (Nirmatelvir/Ritonavir) and Tacrolimus Drug-Drug Interaction in a Kidney Transplant Patient with SARS-2-CoV infection: A Case Report, Transplant Proc
Reed, Rice, Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties, Curr Top Microbiol Immunol
Schrell, Fuchs, Dickmanns, Scheibner, Olejnik et al., Inhibitors of dihydroorotate dehydrogenase synergize with the broad antiviral activity of 4'-fluorouridine, Antiviral Res
Shaban, Muller, Mayr-Buro, Weiser, Meier-Soelch et al., Multi-level inhibition of coronavirus replication by chemical ER stress, Nat Commun
Shin, Ha, Machida, Lee, The stress-inducible ER chaperone GRP78/BiP is upregulated during SARS-CoV-2 infection and acts as a pro-viral protein, Nat Commun
Shin, Toyoda, Fukuhara, Shimomura, a. GRP78, a Novel Host Factor for SARS-CoV-2: The Emerging Roles in COVID-19 Related to Metabolic Risk Factors, Biomedicines
Shin, Toyoda, Nishitani, Fukuhara, Kita et al., Possible Involvement of Adipose Tissue in Patients With Older Age, Obesity, and Diabetes With SARS-CoV-2 Infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of Hyperinsulinemia Management in COVID-19, Diabetes
Shiu, Pouyssegur, Pastan, Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts, Proc Natl Acad Sci U S A
Stegmann, Dickmanns, Gerber, Nikolova, Klemke et al., The folate antagonist methotrexate diminishes replication of the coronavirus SARS-CoV-2 and enhances the antiviral efficacy of remdesivir in cell culture models, Virus research
Stegmann, Dickmanns, Heinen, Blaurock, Karrasch et al., Inhibitors of dihydroorotate dehydrogenase cooperate with molnupiravir and N4-hydroxycytidine to suppress SARS-CoV-2 replication, iScience
Steiner, Kratzel, Barut, Lang, Aguiar Moreira et al., SARS-CoV-2 biology and host interactions, Nat Rev Microbiol
Ton, Pandey, Smith, Ban, Fernandez et al., Targeting SARS-CoV-2 papain-like protease in the postvaccine era, Trends Pharmacol Sci
Vig, Buitinga, Rondas, Crèvecoeur, Van Zandvoort et al., Cytokine-induced translocation of GRP78 to the plasma membrane triggers a pro-apoptotic feedback loop in pancreatic beta cells, Cell Death & Disease
Wolfel, Corman, Guggemos, Seilmaier, Zange et al., Virological assessment of hospitalized patients with COVID-2019, Nature
Xu, Yang, Yang, Berezowska, Gao et al., Endoplasmic Reticulum Stress Signaling as a Therapeutic Target in Malignant Pleural Mesothelioma, Cancers
Yoshida, Okada, Haze, Yanagi, Yura et al., ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response, Mol Cell Biol
Zhang, Liu, Ni, Gill, Lee, Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP, J Biol Chem
Zhu, Lee, Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis, J Cell Physiol
Zibat, Zhang, Dickmanns, Stegmann, Dobbelstein et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience
DOI record:
{
"DOI": "10.1101/2025.03.09.642200",
"URL": "http://dx.doi.org/10.1101/2025.03.09.642200",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:p>Nirmatrelvir, the active compound of the drug Paxlovid, inhibits the Main protease of SARS-CoV-2 (M<jats:sup>Pro</jats:sup>, 3CL<jats:sup>Pro</jats:sup>, NSP5). Its therapeutic application reduces but does not abolish the progression of COVID-19 in humans. Here we report a strong synergy of Nirmatrelvir with inhibitors of the ER chaperone GRP78 (HSPA5, BiP). Combining Nirmatrelvir with the GRP78-antagonizing drug candidate HA15 strongly inhibits the replication of SARS-CoV-2, to a far greater extent than either drug alone, as observed by diminished cytopathic effect, levels of detectable virus RNA, TCID<jats:sub>50</jats:sub>titers, accumulation of the non-structural protein 3 (NSP3), as well as Spike and N proteins. The original SARS-CoV-2 strain as well as an Omicron variant were similarly susceptible towards the drug combination. Other GRP78 inhibitors or siRNAs targeting GRP78 also fortified the antiviral effect of Nirmatrelvir. In a hamster model of COVID-19, the combination of Nirmatrelvir with HA15 alleviated pneumonia-induced pulmonary atelectasis more effectively than the single drugs. In conclusion, inhibition of the virus Main protease and cellular GRP78 cooperatively diminishes virus replication and may improve COVID-19 therapy.</jats:p>",
"accepted": {
"date-parts": [
[
2025,
3,
10
]
]
},
"author": [
{
"affiliation": [],
"family": "Al Krad",
"given": "Doha",
"sequence": "first"
},
{
"affiliation": [],
"family": "Stegmann",
"given": "Kim M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dickmanns",
"given": "Antje",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blaurock",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohl",
"given": "Björn-Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wille",
"given": "Sina Jasmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breithaupt",
"given": "Angele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Britzke",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balkema-Buschmann",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dobbelstein",
"given": "Matthias",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
3,
11
]
],
"date-time": "2025-03-11T12:56:03Z",
"timestamp": 1741697763000
},
"deposited": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T22:30:14Z",
"timestamp": 1741818614000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2025,
3,
12
]
],
"date-time": "2025-03-12T23:10:25Z",
"timestamp": 1741821025400,
"version": "3.38.0"
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
3,
11
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
3,
11
]
],
"date-time": "2025-03-11T00:00:00Z",
"timestamp": 1741651200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2025.03.09.642200",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2025,
3,
11
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2025,
3,
11
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/j.jacc.2022.08.800",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.1"
},
{
"DOI": "10.1002/jmv.28441",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.2"
},
{
"DOI": "10.1128/mBio.00524-13",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.3",
"unstructured": "Angelini, M.M. , Akhlaghpour, M. , Neuman, B.W. , Buchmeier, M.J ., 2013. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. mBio 4."
},
{
"DOI": "10.1038/s41598-022-19222-4",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.4"
},
{
"DOI": "10.1016/j.jbc.2021.100759",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.5",
"unstructured": "Carlos, A.J. , Ha, D.P. , Yeh, D.-W. , Van Krieken, R. , Tseng, C.-C. , Zhang, P. , Gill, P. , Machida, K. , Lee, A.S. , 2021. The chaperone GRP78 is a host auxiliary factor for SARS-CoV-2 and GRP78 depleting antibody blocks viral entry and infection. Journal of Biological Chemistry 296."
},
{
"DOI": "10.3389/fnins.2017.00177",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.6",
"unstructured": "Casas, C ., 2017. GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Frontiers in Neuroscience 11."
},
{
"DOI": "10.1038/s41579-024-01036-y",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.7"
},
{
"DOI": "10.2807/1560-7917.ES.2020.25.3.2000045",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.8",
"unstructured": "Corman, V.M. , Landt, O. , Kaiser, M. , Molenkamp, R. , Meijer, A. , Chu, D.K. , Bleicker, T. , Brunink, S. , Schneider, J. , Schmidt, M.L. , Mulders, D.G. , Haagmans, B.L. , van der Veer, B. , van den Brink, S. , Wijsman, L. , Goderski, G. , Romette, J.L. , Ellis, J. , Zambon, M. , Peiris, M. , Goossens, H. , Reusken, C. , Koopmans, M.P. , Drosten, C. , 2020. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 25."
},
{
"DOI": "10.1126/science.abb4489",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.9"
},
{
"DOI": "10.1038/nrd4201",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.10"
},
{
"DOI": "10.1186/s13578-024-01297-3",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.11"
},
{
"DOI": "10.1183/13993003.congress-2022.2380",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.12"
},
{
"DOI": "10.1016/j.ebiom.2023.104869",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.13"
},
{
"DOI": "10.1038/s41580-020-0250-z",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.14"
},
{
"DOI": "10.1016/j.lfs.2019.04.022",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.15"
},
{
"DOI": "10.1080/15548627.2023.2238579",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.16"
},
{
"DOI": "10.1007/bf01863914",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.17"
},
{
"DOI": "10.1038/s41598-020-79803-z",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.18"
},
{
"DOI": "10.1016/j.ejps.2014.12.017",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.19"
},
{
"DOI": "10.1038/nrc3701",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.20"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.21"
},
{
"DOI": "10.3390/ijms23179866",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.22",
"unstructured": "Loos, N.H.C. , Beijnen, J.H. , Schinkel, A.H ., 2022. The Mechanism-Based Inactivation of CYP3A4 by Ritonavir: What Mechanism? Int J Mol Sci 23."
},
{
"DOI": "10.7554/eLife.72182",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.23",
"unstructured": "Luo, D. , Fan, N. , Zhang, X. , Ngo, F.Y. , Zhao, J. , Zhao, W. , Huang, M. , Li, D. , Wang, Y. , Rong, J. , 2022. Covalent inhibition of endoplasmic reticulum chaperone GRP78 disconnects the transduction of ER stress signals to inflammation and lipid accumulation in diet-induced obese mice. 11."
},
{
"DOI": "10.1038/nrc1505",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.24"
},
{
"DOI": "10.1128/JVI.02274-16",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.25",
"unstructured": "Nain, M. , Mukherjee, S. , Karmakar, S.P. , Paton, A.W. , Paton, J.C. , Abdin, M.Z. , Basu, A. , Kalia, M. , Vrati, S ., 2017. GRP78 Is an Important Host Factor for Japanese Encephalitis Virus Entry and Replication in Mammalian Cells. J Virol 91."
},
{
"DOI": "10.1042/BJ20101569",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.26"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.27"
},
{
"DOI": "10.1111/j.1365-2893.2009.01118.x",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.28"
},
{
"DOI": "10.1016/j.transproceed.2022.04.015",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.29"
},
{
"DOI": "10.1007/978-3-642-59605-6_4",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.30"
},
{
"DOI": "10.1016/j.antiviral.2024.106046",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.31"
},
{
"DOI": "10.1038/s41467-021-25551-1",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.32"
},
{
"DOI": "10.3390/biomedicines10081995",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.33",
"unstructured": "Shin, J. , Toyoda, S. , Fukuhara, A. , Shimomura, I ., 2022a. GRP78, a Novel Host Factor for SARS-CoV-2: The Emerging Roles in COVID-19 Related to Metabolic Risk Factors. Biomedicines 10."
},
{
"DOI": "10.2337/db20-1094",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.34"
},
{
"DOI": "10.1038/s41467-022-34065-3",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.35"
},
{
"DOI": "10.1073/pnas.74.9.3840",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.36"
},
{
"DOI": "10.1016/j.virusres.2021.198469",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.37"
},
{
"DOI": "10.1016/j.isci.2022.104293",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.38"
},
{
"DOI": "10.1038/s41579-023-01003-z",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.39"
},
{
"DOI": "10.1016/j.tips.2022.08.008",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.40"
},
{
"DOI": "10.1038/s41419-019-1518-0",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.41"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.42"
},
{
"DOI": "10.3390/cancers11101502",
"doi-asserted-by": "crossref",
"key": "2025031215300591000_2025.03.09.642200v1.43",
"unstructured": "Xu, D. , Yang, H. , Yang, Z. , Berezowska, S. , Gao, Y. , Liang, S.Q. , Marti, T.M. , Hall, S.R.R. , Dorn, P. , Kocher, G.J. , Schmid, R.A. , Peng, R.W ., 2019. Endoplasmic Reticulum Stress Signaling as a Therapeutic Target in Malignant Pleural Mesothelioma. Cancers 11."
},
{
"DOI": "10.1128/MCB.20.18.6755-6767.2000",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.44"
},
{
"DOI": "10.1074/jbc.M109.087445",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.45"
},
{
"DOI": "10.1002/jcp.24923",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.46"
},
{
"DOI": "10.1016/j.isci.2023.107786",
"doi-asserted-by": "publisher",
"key": "2025031215300591000_2025.03.09.642200v1.47"
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2025.03.09.642200"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "The protease inhibitor Nirmatrelvir synergizes with inhibitors of GRP78 to suppress SARS-CoV-2 replication",
"type": "posted-content"
}