Tolerability of Sarilumab – An anti-interleukin-6 receptor monoclonal antibody is controversial for the management of COVID-19
et al., Current Medicine Research and Practice, doi:10.4103/cmrp.cmrp_71_21, Nov 2021
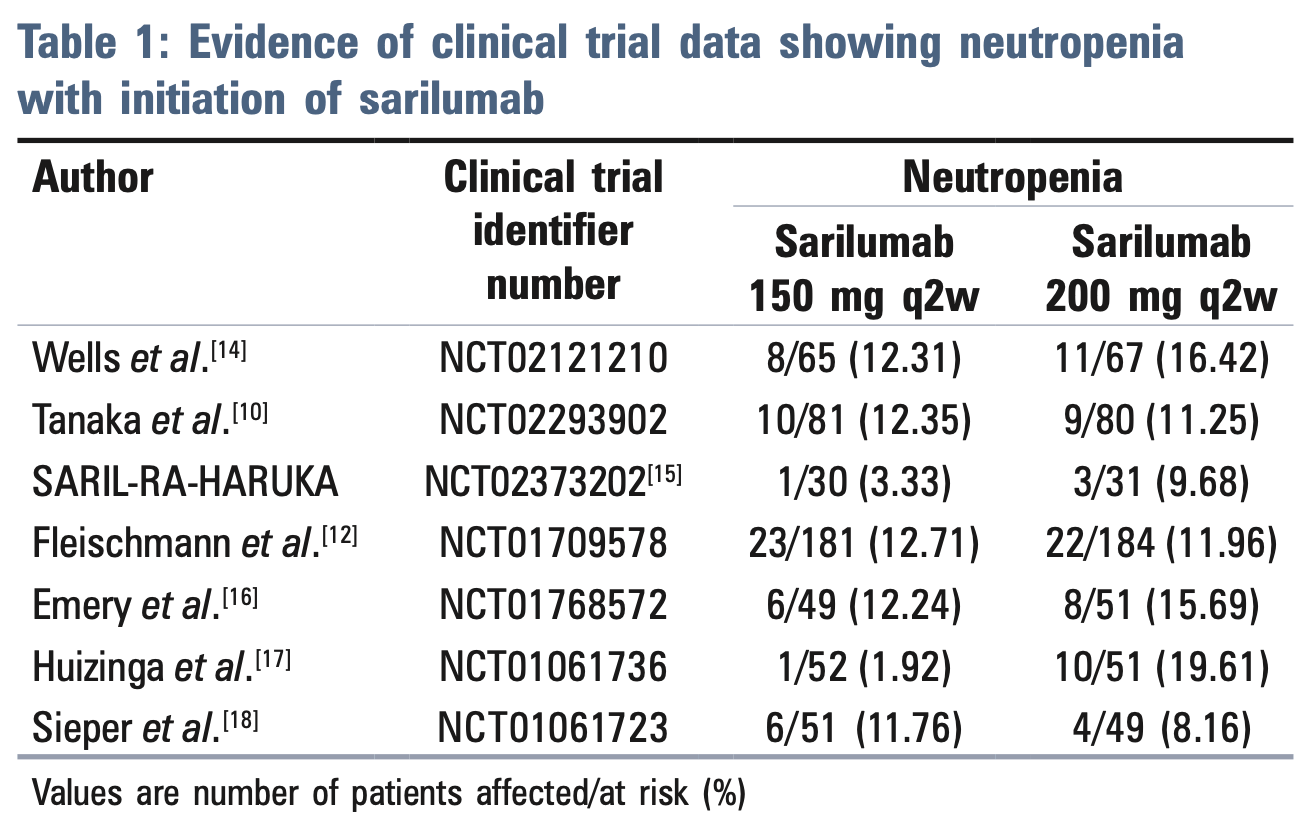
Review of sarilumab, an anti-interleukin-6 receptor monoclonal antibody, for the management of COVID-19. Authors report that sarilumab was proposed as a potential treatment to reduce mortality by abating the inflammatory cytokine storm in severe COVID-19 patients. However, despite having a good safety profile, sarilumab may not be an ideal choice due to higher incidences of infections, neutropenia, and ALT elevation associated with the therapy. Authors find that the risks appear to outweigh the benefits, and sarilumab lacked significant clinical improvement in hospitalized COVID-19 patients.
Abraham et al., 30 Nov 2021, peer-reviewed, 6 authors.
Contact: drrkirankumar@gmail.com.
Tolerability of Sarilumab – An anti-interleukin-6 receptor monoclonal antibody is controversial for the management of COVID-19
Current Medicine Research and Practice, doi:10.4103/cmrp.cmrp_71_21
The emerging upsurge of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) has resulted in a global pandemic which originated in Wuhan city, China. The complicated virology of SARS CoV-2 makes it difficult to evaluate the therapeutic management for this infection. Drug repurposing is been considered in the management of novel coronavirus. Sarilumab, a monoclonal antibody, is being considered in the management of SARS CoV-2 infection, because it binds to both membrane-bound and soluble human interleukin 6 (IL-6) Ra, thereby blocking both IL-6 canonical and trans-signalling pathways. In this study, we tried to evaluate the reasons why Sarilumab is a not suitable option for treating novel coronavirus.
Conflicts of interest There are no conflicts of interest.
References
Burmester, Lin, Patel, Van Adelsberg, Mangan et al., Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): A randomised, double-blind, parallel-group phase III trial, Ann Rheum Dis
Cao, COVID-19: Immunopathology and its implications for therapy, Nat Rev Immunol
Channappanavar, Perlman, Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology, Semin Immunopathol
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study, Lancet
Conti, Ronconi, Caraffa, Gallenga, Ross et al., Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies, J Biol Regul Homeost Agents
Emery, Rondon, Parrino, Lin, Pena-Rossi et al., Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis, Rheumatology
Fleischmann, Van Adelsberg, Lin, Castelar-Pinheiro, Brzezicki et al., Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors, Arthritis Rheumatol
Grilo, Mantalaris, The increasingly human and profitable monoclonal antibody market, Trends Biotechnol
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Huizinga, Fleischmann, Jasson, Radin, Van Adelsberg et al., Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: Efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial, Ann Rheum Dis
Liu, Li, Zhou, Guan, Xiang, Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?, J Autoimmun
Ng, Lee, Yang, Yang, Li et al., Imaging profile of the COVID-19 infection: Radiologic findings and literature review, Radiol Cardiothorac Imaging
Perricone, Triggianese, Bartoloni, Cafaro, Bonifacio et al., The anti-viral facet of anti-rheumatic drugs: Lessons from COVID-19, J Autoimmun
Raimondo, Biggioggero, Crotti, Becciolini, Favalli, Profile of sarilumab and its potential in the treatment of rheumatoid arthritis, Drug Des Devel Ther
Russell, Moss, George, Santaolalla, Cope et al., Associations between immune-suppressive and stimulating drugs and novel COVID-19 -A systematic review of current evidence, Ecancermedicalscience
Semerano, Thiolat, Minichiello, Clavel, Bessis et al., Targeting IL-6 for the treatment of rheumatoid arthritis: Phase II investigational drugs, Expert Opin Investig Drugs
Sieper, Braun, Kay, Badalamenti, Radin et al., Sarilumab for the treatment of ankylosing spondylitis: Results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN), Ann Rheum Dis
Tanaka, Wada, Takahashi, Hagino, Van Hoogstraten et al., Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a randomized, placebo-controlled phase III trial in Japan, Arthritis Res Ther
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Wells, Parrino, Mangan, Paccaly, Lin et al., Immunogenicity of sarilumab monotherapy in patients with rheumatoid arthritis who were inadequate responders or intolerant to disease-modifying antirheumatic drugs, Rheumatol Ther
Zhang, Wu, Li, Zhao, Wang, Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality, Int J Antimicrob Agents
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.4103/cmrp.cmrp_71_21",
"ISSN": [
"2352-0817"
],
"URL": "http://dx.doi.org/10.4103/cmrp.cmrp_71_21",
"abstract": "<jats:sec>\n <jats:title/>\n <jats:p>The emerging upsurge of severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) has resulted in a global pandemic which originated in Wuhan city, China. The complicated virology of SARS CoV-2 makes it difficult to evaluate the therapeutic management for this infection. Drug repurposing is been considered in the management of novel coronavirus. Sarilumab, a monoclonal antibody, is being considered in the management of SARS CoV-2 infection, because it binds to both membrane-bound and soluble human interleukin 6 (IL-6) Ra, thereby blocking both IL-6 canonical and trans-signalling pathways. In this study, we tried to evaluate the reasons why Sarilumab is a not suitable option for treating novel coronavirus.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [],
"family": "Abraham",
"given": "Justin Jacob",
"sequence": "first"
},
{
"affiliation": [],
"family": "Michelle",
"given": "I Jerlin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shevaani",
"given": "S. A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rathinam",
"given": "Kiran Kumar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajanandh",
"given": "Muhasaparur Ganesan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahalingam",
"given": "Vijayakumar Thangavel",
"sequence": "additional"
}
],
"container-title": "Current Medicine Research and Practice",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"lww.com",
"ovid.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
31
]
],
"date-time": "2021-12-31T10:44:55Z",
"timestamp": 1640947495000
},
"deposited": {
"date-parts": [
[
2024,
9,
1
]
],
"date-time": "2024-09-01T22:06:28Z",
"timestamp": 1725228388000
},
"indexed": {
"date-parts": [
[
2024,
9,
2
]
],
"date-time": "2024-09-02T00:04:38Z",
"timestamp": 1725235478114
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
11
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.4103/cmrp.cmrp_71_21",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2581",
"original-title": [],
"page": "280-283",
"prefix": "10.4103",
"published": {
"date-parts": [
[
2021,
11
]
]
},
"published-print": {
"date-parts": [
[
2021,
11
]
]
},
"publisher": "Medknow",
"reference": [
{
"DOI": "10.2147/DDDT.S100302",
"article-title": "Profile of sarilumab and its potential in the treatment of rheumatoid arthritis.",
"author": "Raimondo",
"doi-asserted-by": "crossref",
"first-page": "1593",
"journal-title": "Drug Des Devel Ther",
"key": "R1-5-20240901",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1038/s41577-020-0308-3",
"article-title": "COVID-19: Immunopathology and its implications for therapy.",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Nat Rev Immunol",
"key": "R2-5-20240901",
"volume": "20",
"year": "2020"
},
{
"article-title": "Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies.",
"author": "Conti",
"first-page": "327",
"journal-title": "J Biol Regul Homeost Agents",
"key": "R3-5-20240901",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102468",
"article-title": "The anti-viral facet of anti-rheumatic drugs: Lessons from COVID-19.",
"author": "Perricone",
"doi-asserted-by": "crossref",
"first-page": "102468",
"journal-title": "J Autoimmun",
"key": "R4-5-20240901",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105954",
"article-title": "Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality.",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "105954",
"journal-title": "Int J Antimicrob Agents",
"key": "R5-5-20240901",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.jaut.2020.102452",
"article-title": "Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)?",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "102452",
"journal-title": "J Autoimmun",
"key": "R6-5-20240901",
"volume": "111",
"year": "2020"
},
{
"DOI": "10.3332/ecancer.2020.1022",
"article-title": "Associations between immune-suppressive and stimulating drugs and novel COVID-19 - A systematic review of current evidence.",
"author": "Russell",
"doi-asserted-by": "crossref",
"first-page": "1022",
"journal-title": "Ecancermedicalscience",
"key": "R7-5-20240901",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1517/13543784.2014.912276",
"article-title": "Targeting IL-6 for the treatment of rheumatoid arthritis: Phase II investigational drugs.",
"author": "Semerano",
"doi-asserted-by": "crossref",
"first-page": "979",
"journal-title": "Expert Opin Investig Drugs",
"key": "R8-5-20240901",
"volume": "23",
"year": "2014"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"article-title": "Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology.",
"author": "Channappanavar",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Semin Immunopathol",
"key": "R9-5-20240901",
"volume": "39",
"year": "2017"
},
{
"DOI": "10.1186/s13075-019-1856-4",
"article-title": "Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: Results of a randomized, placebo-controlled phase III trial in Japan.",
"author": "Tanaka",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Arthritis Res Ther",
"key": "R10-5-20240901",
"volume": "21",
"year": "2019"
},
{
"DOI": "10.1136/annrheumdis-2016-210310",
"article-title": "Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): A randomised, double-blind, parallel-group phase III trial.",
"author": "Burmester",
"doi-asserted-by": "crossref",
"first-page": "840",
"journal-title": "Ann Rheum Dis",
"key": "R11-5-20240901",
"volume": "76",
"year": "2017"
},
{
"DOI": "10.1002/art.39944",
"article-title": "Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors.",
"author": "Fleischmann",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Arthritis Rheumatol",
"key": "R12-5-20240901",
"volume": "69",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study.",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "R13-5-20240901",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s40744-019-0157-3",
"article-title": "Immunogenicity of sarilumab monotherapy in patients with rheumatoid arthritis who were inadequate responders or intolerant to disease-modifying antirheumatic drugs.",
"author": "Wells",
"doi-asserted-by": "crossref",
"first-page": "339",
"journal-title": "Rheumatol Ther",
"key": "R14-5-20240901",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.1093/rheumatology/key361",
"article-title": "Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis.",
"author": "Emery",
"doi-asserted-by": "crossref",
"first-page": "849",
"journal-title": "Rheumatology (Oxford",
"key": "R16-5-20240901",
"volume": "58",
"year": "2019"
},
{
"DOI": "10.1136/annrheumdis-2013-204405",
"article-title": "Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: Efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial.",
"author": "Huizinga",
"doi-asserted-by": "crossref",
"first-page": "1626",
"journal-title": "Ann Rheum Dis",
"key": "R17-5-20240901",
"volume": "73",
"year": "2014"
},
{
"DOI": "10.1136/annrheumdis-2013-204963",
"article-title": "Sarilumab for the treatment of ankylosing spondylitis: Results of a Phase II, randomised, double-blind, placebo-controlled study (ALIGN).",
"author": "Sieper",
"doi-asserted-by": "crossref",
"first-page": "1051",
"journal-title": "Ann Rheum Dis",
"key": "R18-5-20240901",
"volume": "74",
"year": "2015"
},
{
"DOI": "10.1016/j.tibtech.2018.05.014",
"article-title": "The increasingly human and profitable monoclonal antibody market.",
"author": "Grilo",
"doi-asserted-by": "crossref",
"first-page": "9",
"journal-title": "Trends Biotechnol",
"key": "R19-5-20240901",
"volume": "37",
"year": "2019"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China.",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "R20-5-20240901",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study.",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "R21-5-20240901",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "R22-5-20240901",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1148/ryct.2020200034",
"article-title": "Imaging profile of the COVID-19 infection: Radiologic findings and literature review.",
"author": "Ng",
"doi-asserted-by": "crossref",
"first-page": "e200034",
"journal-title": "Radiol Cardiothorac Imaging",
"key": "R23-5-20240901",
"volume": "2",
"year": "2020"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.4103/cmrp.cmrp_71_21"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tolerability of Sarilumab – An anti-interleukin-6 receptor monoclonal antibody is controversial for the management of COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1097/lww.0000000000001000",
"volume": "11"
}