Safety, Tolerability, and Pharmacokinetics of Intravenous Doses of PF‐07304814, a Phosphate Prodrug Protease Inhibitor for the Treatment of SARS‐CoV‐2, in Healthy Adult Participants
et al., Clinical Pharmacology in Drug Development, doi:10.1002/cpdd.1174, NCT04627532, Oct 2022
Phase 1 randomized, double-blind, placebo-controlled study of 15 healthy participants showing safety and tolerability of single ascending 24-hour IV infusions of lufotrelvir (PF-07304814), a phosphate prodrug protease inhibitor targeting the 3CL protease of SARS-CoV-2, at doses of 50 to 700 mg. No serious adverse events, treatment discontinuations or deaths were reported, and all adverse events were mild. Dose-proportional pharmacokinetics of the active moiety PF-00835231 were observed, with rapid conversion from the prodrug and sustained plasma concentrations during the infusion period. The results suggest a daily dose of 270-350 mg administered as a 24-hour continuous infusion is expected to maintain the steady-state concentration of PF-00835231 at potentially therapeutic levels.
Zhu et al., 26 Oct 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 11 authors, study period 23 October, 2020 - 17 December, 2020, trial NCT04627532 (history).
Contact: tong.zhu@pfizer.com.
Safety, Tolerability, and Pharmacokinetics of Intravenous Doses of PF‐07304814, a Phosphate Prodrug Protease Inhibitor for the Treatment of SARS‐CoV‐2, in Healthy Adult Participants
Clinical Pharmacology in Drug Development, doi:10.1002/cpdd.1174
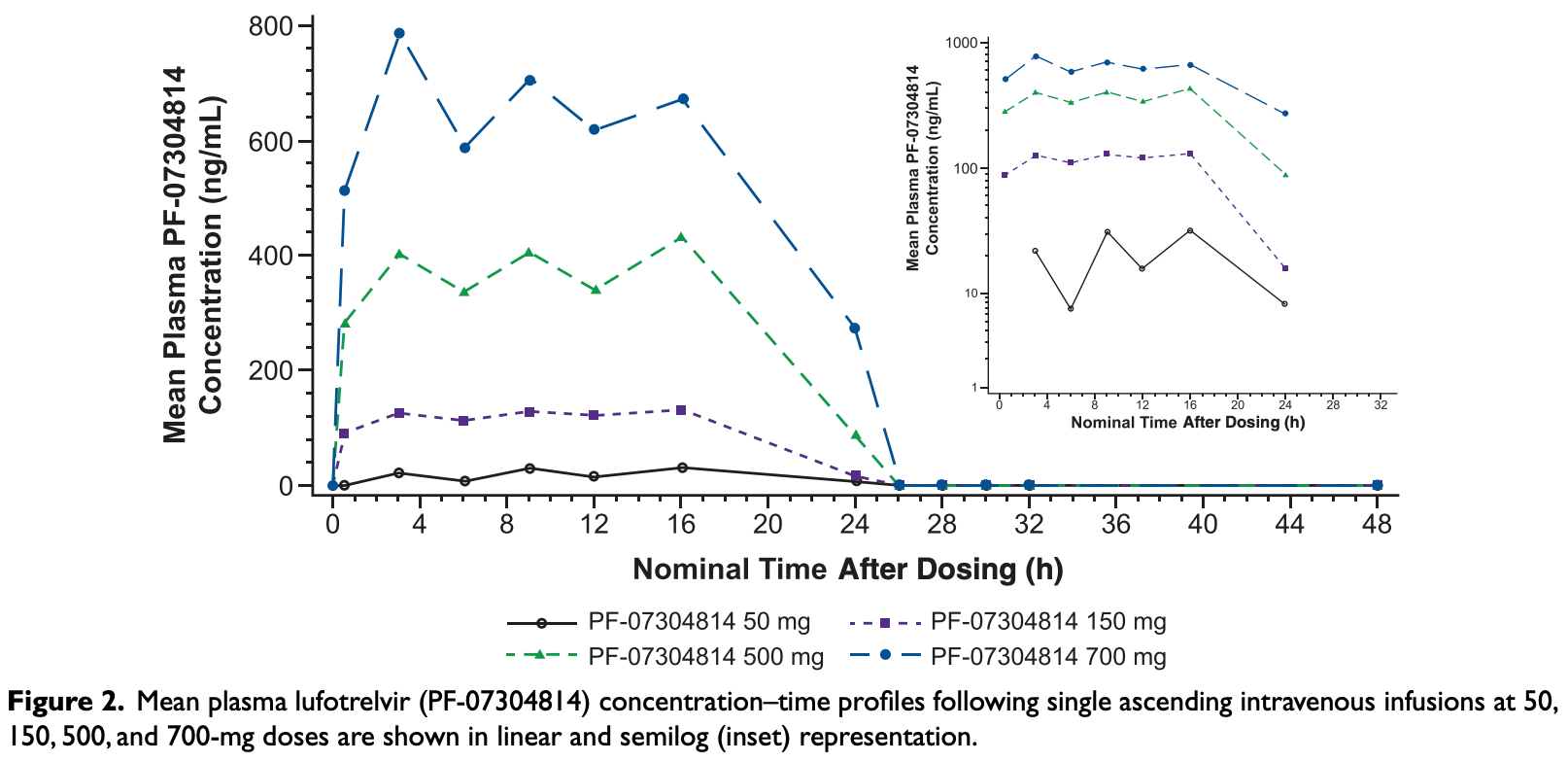
Studies on targeted antivirals for treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the ongoing pandemic, are limited. PF-07304814 (lufotrelvir) is the phosphate prodrug of PF-00835231, a protease inhibitor targeting the 3C-like protease of SARS-CoV-2. This phase 1 study evaluated the safety, tolerability, and pharmacokinetics (PK) of single ascending intravenous doses of lufotrelvir (continuous 24-hour infusion of 50, 150, 500, or 700 mg) versus placebo in healthy volunteers (2 interleaving cohorts: 1, n = 8; 2, n = 7). Each dosing period was separated by a washout interval (≥5 days). Treatment-emergent adverse events, PK, and biomarker concentrations were estimated from plasma/urine samples. Lufotrelvir was administered to 15 volunteers (mean [SD] age 39.7 [11.8] years). No serious adverse events, discontinuations, or deaths were reported. Mean maximum observed concentration of PF-00835231 (active moiety; 97.0 ng/mL to 1288 ng/mL) were observed between median time to maximum concentration of 14 to 16 hours after the start of the lufotrelvir infusion. Near-maximum plasma concentrations of PF-00835231 were observed ≈6 hours after infusion start and sustained until infusion end. PF-00835231 plasma concentrations declined rapidly after infusion end (mean terminal half-life: 500 mg, 2.0 hours; 700 mg, 1.7 hours). Approximately 9%-11% of the dose was recovered in urine as PF-00835231 across doses. A continuous, single-dose, 24-hour infusion of lufotrelvir (50-700 mg) was rapidly converted to PF-00835231 (active moiety), with dose-proportional PK exposures and no significant safety concerns. A daily, 24-hour continuous infusion of 270 to 350 mg is expected to maintain PF-00835231 concentration at steady state/above effective antiviral concentrations. Further studies exploring lufotrelvir efficacy in patients with coronavirus disease 2019 are ongoing.
After Dosing (h) After Dosing (h)
Conflicts of Interest All authors are employees of Pfizer, Inc. and may hold stock/stock options in Pfizer.
References
Adamsick, Gandhi, Bidell, Remdesivir in patients with acute or chronic kidney disease and COVID-19, J Am Soc Nephrol
Anirudhan, Lee, Cheng, Cooper, Rong, Targeting SARS-CoV-2 viral proteases as a therapeutic strategy to treat COVID-19, J Med Virol
Baden, Sahly, Essink, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N Engl J Med
Baig, Sharma, Ahmad, Abohashrh, Alam et al., Is PF-00835231 a pan-SARS-CoV-2 M pro inhibitor? A comparative study, Molecules
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19 -final report, New England Journal of Medicine
Bergwerk, Gonen, Lustig, COVID-19 breakthrough infections in vaccinated health care workers, N Engl J Med
Boras, Jones, Anson, Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID-19, Nat Commun
Chaar, Makuch, Emergency use authorization for remdesivir and its potential implications, Ther Innov Regul Sci
Chu, Chan, Evers, Identification of endogenous biomarkers to predict the propensity of drug candidates to cause hepatic or renal transporter-mediated drug-drug interactions, J Pharm Sci
De Vries, Prescott, A comparative analysis of SARS-CoV-2 antivirals characterizes 3CL pro inhibitor PF-00835231 as a potential new treatment for covid-19, J Virol
Dn, dose normalized AUC last ; C 24 , concentration at 24 hours; C max , maximum observed concentration; C ss , concentration at steady state; dn, dose normalized; N, number of participants in the treatment group contributing to summary statistic; NC, not calculated; NR, not reported; PK, pharmacokinetics; SD, standard deviation; t 1/2 , terminal half-life; t max , time to maximum concentration. a Geometric mean (geometric % coefficient of variation) and arithmetic mean
Dn, h/mL/mg NC, NC NC
Dorr, Westby, Dobbs, Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity, Antimicrob Agents Chemother
Fan, Wei, Feng, Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase, J Biol Chem
Fan, Zhang, Ma, Zhang, Safety profile of the antiviral drug remdesivir: An update, Biomed Pharmacother
Forni, Mantovani, Covid-19 Commission of Accademia Nazionale dei Lincei R. COVID-19 vaccines: Where we stand and challenges ahead, Cell Death Differ
Hoffman, Kania, Brothers, Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19, Journal of Medicinal Chemistry
Kow, Aldeyab, Hasan, Effect of remdesivir on mortality in patients with COVID-19: A metaanalysis of randomized control trials, J Med Virol
Kunze, Ediage, Dillen, Monshouwer, Snoeys, Clinical investigation of coproporphyrins as sensitive biomarkers to predict mild to strong OATP1B-mediated drug-drug interactions, Clin Pharmacokinet
Mengist, Mekonnen, Mohammed, Shi, Potency, safety, and pharmacokinetic profiles of potential inhibitors targeting SARS-CoV-2 main protease, Front Pharmacol
Mouffak, Shubbar, Saleh, El-Awady, Recent advances in management of COVID-19: A review, Biomed Pharmacother
Muramatsu, Takemoto, Kim, Wiley Online Library for rules of use; OA articles are governed by the applicable Creative Commons License by novel subsite cooperativity, Proceedings of the National Academy of Sciences
Nathan, Shawa, De, Torre, A narrative review of the clinical practicalities of bamlanivimab and etesevimab antibody therapies for SARS-CoV-2, Infect Dis Ther
Parasher, COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment, Postgraduate Medical Journal
Peter, Sandeep, Rao, Kalpana, Calming the storm: Natural immunosuppressants as adjuvants to target the cytokine storm in COVID-19, Front Pharmacol
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Reddy, Morcos, Pogam, Pharmacokinetic/pharmacodynamic predictors of clinical potency for hepatitis c virus nonnucleoside polymerase and protease inhibitors, Antimicrob Agents Chemother
Regas, Culla, Bellfill, Adverse reactions of drugs specifically used for treatment of SARS-CoV-2 infection, Med Clin (Engl Ed)
Saint-Raymond, Sato, Kishioka, Teixeira, Hasslboeck et al., Remdesivir emergency approvals: A comparison of the U.S., Japanese, and EU systems, Expert Rev Clin Pharmacol
Sanyaolu, Okorie, Marinkovic, Comorbidity and its impact on patients with COVID-19, SN Compr Clin Med
Shen, Peterson, Sedaghat, Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs, Nat Med
Ss, mL/mg 1, 1
Ziebuhr, Herold, Siddell, Characterization of a human coronavirus (strain 229e) 3C-like proteinase activity, J Virol
DOI record:
{
"DOI": "10.1002/cpdd.1174",
"ISSN": [
"2160-763X",
"2160-7648"
],
"URL": "http://dx.doi.org/10.1002/cpdd.1174",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Studies on targeted antivirals for treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the cause of the ongoing pandemic, are limited. PF‐07304814 (lufotrelvir) is the phosphate prodrug of PF‐00835231, a protease inhibitor targeting the 3C‐like protease of SARS‐CoV‐2. This phase 1 study evaluated the safety, tolerability, and pharmacokinetics (PK) of single ascending intravenous doses of lufotrelvir (continuous 24‐hour infusion of 50, 150, 500, or 700 mg) versus placebo in healthy volunteers (2 interleaving cohorts: 1, n = 8; 2, n = 7). Each dosing period was separated by a washout interval (≥5 days). Treatment‐emergent adverse events, PK, and biomarker concentrations were estimated from plasma/urine samples. Lufotrelvir was administered to 15 volunteers (mean [SD] age 39.7 [11.8] years). No serious adverse events, discontinuations, or deaths were reported. Mean maximum observed concentration of PF‐00835231 (active moiety; 97.0 ng/mL to 1288 ng/mL) were observed between median time to maximum concentration of 14 to 16 hours after the start of the lufotrelvir infusion. Near‐maximum plasma concentrations of PF‐00835231 were observed ≈6 hours after infusion start and sustained until infusion end. PF‐00835231 plasma concentrations declined rapidly after infusion end (mean terminal half‐life: 500 mg, 2.0 hours; 700 mg, 1.7 hours). Approximately 9%–11% of the dose was recovered in urine as PF‐00835231 across doses. A continuous, single‐dose, 24‐hour infusion of lufotrelvir (50–700 mg) was rapidly converted to PF‐00835231 (active moiety), with dose‐proportional PK exposures and no significant safety concerns. A daily, 24‐hour continuous infusion of 270 to 350 mg is expected to maintain PF‐00835231 concentration at steady state/above effective antiviral concentrations. Further studies exploring lufotrelvir efficacy in patients with coronavirus disease 2019 are ongoing.</jats:p>",
"alternative-id": [
"10.1002/cpdd.1174"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-02-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-08-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-10-26"
}
],
"author": [
{
"affiliation": [
{
"name": "Pfizer Worldwide Research Development and Medical Cambridge Massachusetts USA"
}
],
"family": "Zhu",
"given": "Tong",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Pfizer Clinical Research Unit New Haven Connecticut USA"
}
],
"family": "Pawlak",
"given": "Sylvester",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Worldwide Research Development and Medical, Pearl River New York USA"
}
],
"family": "Toussi",
"given": "Sima S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Worldwide Research Development and Medical Cambridge UK"
}
],
"family": "Hackman",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Clinical Research Unit New Haven Connecticut USA"
}
],
"family": "Thompson",
"given": "Kimberly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Worldwide Research Development and Medical Groton Connecticut USA"
}
],
"family": "Song",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Global Product Development Groton Connecticut USA"
}
],
"family": "Salageanu",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Global Product Development Groton Connecticut USA"
}
],
"family": "Winter",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Global Product Development Groton Connecticut USA"
}
],
"family": "Shi",
"given": "Haihong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Global Product Development Groton Connecticut USA"
}
],
"family": "Winton",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pfizer Worldwide Research Development and Medical Cambridge Massachusetts USA"
}
],
"family": "Binks",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "Clinical Pharmacology in Drug Development",
"container-title-short": "Clinical Pharm in Drug Dev",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"accp1.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
10,
26
]
],
"date-time": "2022-10-26T08:09:53Z",
"timestamp": 1666771793000
},
"deposited": {
"date-parts": [
[
2023,
8,
17
]
],
"date-time": "2023-08-17T05:35:52Z",
"timestamp": 1692250552000
},
"indexed": {
"date-parts": [
[
2024,
3,
7
]
],
"date-time": "2024-03-07T20:43:08Z",
"timestamp": 1709844188613
},
"is-referenced-by-count": 4,
"issue": "12",
"issued": {
"date-parts": [
[
2022,
10,
26
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
10,
26
]
],
"date-time": "2022-10-26T00:00:00Z",
"timestamp": 1666742400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpdd.1174",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cpdd.1174",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://accp1.onlinelibrary.wiley.com/doi/pdf/10.1002/cpdd.1174",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1382-1393",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
10,
26
]
]
},
"published-online": {
"date-parts": [
[
2022,
10,
26
]
]
},
"published-print": {
"date-parts": [
[
2022,
12
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1136/postgradmedj-2020-138577",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"key": "e_1_2_10_3_1",
"unstructured": "Johns Hopkins coronavirus resource centre.https://coronavirus.jhu.edu/map.html. Accessed May 18 2021."
},
{
"article-title": "Comorbidity and its impact on patients with COVID‐19",
"author": "Sanyaolu A",
"first-page": "1",
"journal-title": "SN Compr Clin Med",
"key": "e_1_2_10_4_1",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.583777",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.1038/s41418-020-00720-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1056/NEJMoa2109072",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1016/j.biopha.2021.112107",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"key": "e_1_2_10_11_1",
"unstructured": "WHO Guidelines Review Committee.Therapeutics and COVID‐19: Living guideline. Geneva: World Health Organization;2022."
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1080/17512433.2020.1821650",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1007/s43441-020-00212-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1681/ASN.2020050589",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1002/jmv.26638",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.1128/JVI.01819-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.1007/s40121-021-00515-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1073/pnas.1601327113",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1128/jvi.69.7.4331-4338.1995",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1074/jbc.M310875200",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1021/acs.jmedchem.0c01063",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1002/jmv.26814",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.1038/s41467-021-26239-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.3390/molecules26061678",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1016/j.xphs.2017.04.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1007/s40262-018-0648-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1128/AAC.49.11.4721-4732.2005",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_28_1"
},
{
"DOI": "10.1128/AAC.06283-11",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_29_1"
},
{
"DOI": "10.1038/nm1777",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_30_1"
},
{
"DOI": "10.1016/j.medcle.2020.06.026",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1016/j.biopha.2020.110532",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.3389/fphar.2020.630500",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"key": "e_1_2_10_34_1",
"unstructured": "First‐in‐human study to evaluate safety tolerability and pharmacokinetics following single ascending and multiple ascending doses of PF‐07304814 in hospitalized participants with COVID‐19.https://clinicaltrials.gov/ct2/show/study/NCT04535167?term=PF-07304814&cond=Covid19&draw=2&rank=1. Accessed April 3 2021. "
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://accp1.onlinelibrary.wiley.com/doi/10.1002/cpdd.1174"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Safety, Tolerability, and Pharmacokinetics of Intravenous Doses of PF‐07304814, a Phosphate Prodrug Protease Inhibitor for the Treatment of SARS‐CoV‐2, in Healthy Adult Participants",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "11"
}