Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2023.1204297, Nov 2023
Budesonide for COVID-19
28th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
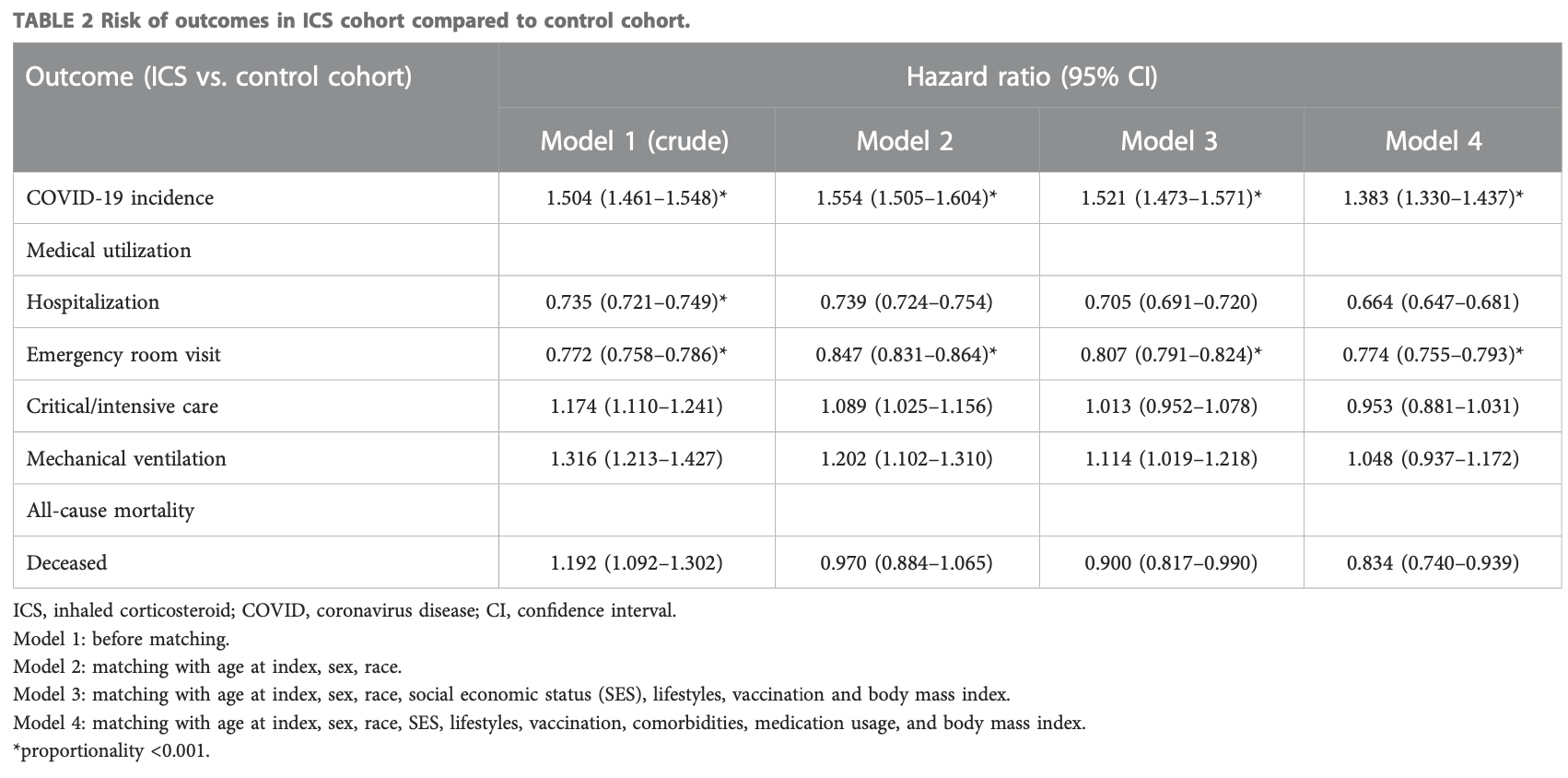
PSM retrospective 64,587 asthmatic patients using inhaled corticosteroids (ICS) and 64,587 matched controls without ICS use. ICS medications included budesonide, mometasone, flunisolide, beclomethasone, fluticasone, and ciclesonide. ICS use was associated with a higher risk of COVID-19 infection (hazard ratio 1.383) but lower risks of hospitalization (HR 0.664), emergency department visits (HR 0.774), and mortality (HR 0.834).
Yong et al., 1 Nov 2023, retrospective, USA, peer-reviewed, 6 authors, study period January 2020 - December 2022.
Contact: shiowing0107@gmail.com, jccwei@gmail.com.
Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid
Frontiers in Pharmacology, doi:10.3389/fphar.2023.1204297
Background: The impact of inhaled corticosteroid (ICS) in the interaction between asthma, COVID-19 and COVID-19 associated outcomes remain largely unknown. The objective of this study is to investigate the risk of COVID-19 and its related outcomes in patients with asthma using and not using inhaled corticosteroid (ICS). Methods: We used the TriNetX Network, a global federated network that comprises 55 healthcare organizations (HCO) in the United States, to conduct a retrospective cohort study. Patients with a diagnosis of asthma with and without ICS between January 2020 and December 2022 were included. Propensity score matching was used to match the case cohorts. Risks of COVID-19 incidence and medical utilizations were evaluated. Results: Out of 64,587 asthmatic patients with ICS and without ICS, asthmatic patients with ICS had a higher incidence of COVID-19 (Hazard ratio, HR: 1.383, 95% confidence interval, CI: 1.330-1.437). On the contrary, asthmatic patients with ICS revealed a significantly lower risk of hospitalization (HR: 0.664, 95% CI: 0.647-0.681), emergency department visits (HR: 0.774, 95% CI: 0.755-0.793), and mortality (HR:0.834, 95% CI:0.740-0.939). In addition, subgroup or sensitivity analyses were also conducted to examine the result of different vaccination status, disease severity, or COVID-19 virus variants.
Conclusion: For asthmatic patients using ICS, risk of COVID-19 was significantly higher than non-users. The observed association could provide potential guidance for primary care physicians regarding the risk of COVID-19 in asthmatic patients.
Ethics statement The studies involving humans were approved by the Institutional Review Board of Chung Shan Medical University Hospital (CSMUH No: CS2-21176) . The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions Study conception and design: S-BY, C-WT, S-IW, C-JL, S-YG, and J-CW. Data acquisition: S-BY and J-CW. Data analysis and demonstration: S-BY, C-WT, S-IW, and S-YG. Original draft preparation: S-BY, C-WT, S-IW, S-YG, C-JL, and J-CW. All authors contributed to the article and approved the submitted version.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at:..
References
Abutiban, Saleh, Hayat, Tarakmah, Al-Herz et al., COVID-19 outcomes among rheumatic disease patients in Kuwait: data from the COVID-19 Global Rheumatology Alliance (C19-GRA) physician registry, Int. J. Rheum. Dis, doi:10.1111/1756-185X.14332
Adir, Fireman Klein, Saliba, Inhaled corticosteroids and COVID-19 outcomes in asthma: the Israeli experience, ERJ Open Res, doi:10.1183/23120541.00014-2022
Aveyard, Gao, Lindson, Hartmann-Boyce, Watkinson et al., Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00095-3
Bloom, Drake, Docherty, Lipworth, Johnston et al., Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK, Lancet Respir. Med, doi:10.1016/S2213-2600(21)00013-8
Choi, Park, Lee, Suh, Song et al., Effect of asthma and asthma medication on the prognosis of patients with COVID-19, Eur. Respir. J, doi:10.1183/13993003.02226-2020
Convertino, Tuccori, Ferraro, Valdiserra, Cappello et al., Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients, Crit. Care, doi:10.1186/s13054-020-03020-3
Dolby, Nafilyan, Morgan, Kallis, Sheikh et al., Relationship between asthma and severe COVID-19: a national cohort study, Thorax, doi:10.1136/thoraxjnl-2021-218629
Eger, Bel, Asthma and COVID-19: do we finally have answers?, Eur. Respir. J, doi:10.1183/13993003.04451-2020
Gif, Global Strategy for asthma management and prevention
Green, Merzon, Vinker, Golan-Cohen, COVID-19 Susceptibility in Bronchial asthma, J. Allergy Clin. Immunol. Pract, doi:10.1016/j.jaip.2020.11.020
Hasan, Capstick, Zaidi, Kow, Merchant, Use of corticosteroids in asthma and COPD patients with or without COVID-19, Respir. Med, doi:10.1016/j.rmed.2020.106045
Izquierdo, Almonacid, Gonzalez, Del Rio-Bermudez, Ancochea et al., The impact of COVID-19 on patients with asthma, Eur. Respir. J, doi:10.1183/13993003.03142-2020
Kahn, Callahan, Barnard, Bauck, Brown et al., A Harmonized data quality assessment Terminology and framework for the Secondary Use of electronic health record data, EGEMS (Wash DC), doi:10.13063/2327-9214.1244
Kim, Rhee, Shim, Park, Yoo et al., Inhaled corticosteroids in asthma and the risk of pneumonia, Allergy Asthma Immunol. Res, doi:10.4168/aair.2019.11.6.795
Paljarvi, Forton, Luciano, Herttua, Fazel, Analysis of neuropsychiatric diagnoses after montelukast initiation, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2022.13643
Papadopoulos, Custovic, Deschildre, Mathioudakis, Phipatanakul et al., Impact of COVID-19 on pediatric asthma: practice adjustments and disease burden, J. Allergy Clin. Immunol. Pract, doi:10.1016/j.jaip.2020.06.001
Peters, Sajuthi, Deford, Christenson, Rios et al., COVID-19-related genes in sputum cells in asthma. relationship to demographic features and corticosteroids, Am. J. Respir. Crit. Care Med, doi:10.1164/rccm.202003-0821OC
Schultze, Walker, Mackenna, Morton, Bhaskaran et al., Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30415-X
Skevaki, Karsonova, Karaulov, Xie, Renz, Asthmaassociated risk for COVID-19 development, J. Allergy Clin. Immunol, doi:10.1016/j.jaci.2020.09.017
Taquet, Sillett, Zhu, Mendel, Camplisson et al., Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients, Lancet Psychiatry, doi:10.1016/S2215-0366(22)00260-7
Thompson, Burgess, Naleway, Tyner, Yoon et al., Prevention and attenuation of covid-19 with the BNT162b2 and mRNA-1273 vaccines, N. Engl. J. Med, doi:10.1056/NEJMoa2107058
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
Yang, Zhang, Chen, Lin, Zeng et al., Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis, Infection, doi:10.1007/s15010-018-1229-y
DOI record:
{
"DOI": "10.3389/fphar.2023.1204297",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2023.1204297",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> The impact of inhaled corticosteroid (ICS) in the interaction between asthma, COVID-19 and COVID-19 associated outcomes remain largely unknown. The objective of this study is to investigate the risk of COVID-19 and its related outcomes in patients with asthma using and not using inhaled corticosteroid (ICS).</jats:p><jats:p><jats:bold>Methods:</jats:bold> We used the TriNetX Network, a global federated network that comprises 55 healthcare organizations (HCO) in the United States, to conduct a retrospective cohort study. Patients with a diagnosis of asthma with and without ICS between January 2020 and December 2022 were included. Propensity score matching was used to match the case cohorts. Risks of COVID-19 incidence and medical utilizations were evaluated.</jats:p><jats:p><jats:bold>Results:</jats:bold> Out of 64,587 asthmatic patients with ICS and without ICS, asthmatic patients with ICS had a higher incidence of COVID-19 (Hazard ratio, HR: 1.383, 95% confidence interval, CI: 1.330–1.437). On the contrary, asthmatic patients with ICS revealed a significantly lower risk of hospitalization (HR: 0.664, 95% CI: 0.647–0.681), emergency department visits (HR: 0.774, 95% CI: 0.755–0.793), and mortality (HR:0.834, 95% CI:0.740–0.939). In addition, subgroup or sensitivity analyses were also conducted to examine the result of different vaccination status, disease severity, or COVID-19 virus variants.</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> For asthmatic patients using ICS, risk of COVID-19 was significantly higher than non-users. The observed association could provide potential guidance for primary care physicians regarding the risk of COVID-19 in asthmatic patients.</jats:p>",
"alternative-id": [
"10.3389/fphar.2023.1204297"
],
"author": [
{
"affiliation": [],
"family": "Yong",
"given": "Su-Boon",
"sequence": "first"
},
{
"affiliation": [],
"family": "Gau",
"given": "Shuo-Yan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Chia-Jung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tseng",
"given": "Chih-Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Shiow-Ing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wei",
"given": "James Cheng-Chung",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T11:03:27Z",
"timestamp": 1698836607000
},
"deposited": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T11:03:31Z",
"timestamp": 1698836611000
},
"indexed": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T00:40:55Z",
"timestamp": 1698885655600
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
1
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
1
]
],
"date-time": "2023-11-01T00:00:00Z",
"timestamp": 1698796800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1204297/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
11,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
1
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1111/1756-185X.14332",
"article-title": "COVID-19 outcomes among rheumatic disease patients in Kuwait: data from the COVID-19 Global Rheumatology Alliance (C19-GRA) physician registry",
"author": "Abutiban",
"doi-asserted-by": "publisher",
"first-page": "743",
"journal-title": "Int. J. Rheum. Dis.",
"key": "B1",
"volume": "25",
"year": "2022"
},
{
"DOI": "10.1183/23120541.00014-2022",
"article-title": "Inhaled corticosteroids and COVID-19 outcomes in asthma: the Israeli experience",
"author": "Adir",
"doi-asserted-by": "publisher",
"first-page": "00014-2022",
"journal-title": "ERJ Open Res.",
"key": "B2",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(21)00095-3",
"article-title": "Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study",
"author": "Aveyard",
"doi-asserted-by": "publisher",
"first-page": "909",
"journal-title": "Lancet Respir. Med.",
"key": "B3",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00013-8",
"article-title": "Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK",
"author": "Bloom",
"doi-asserted-by": "publisher",
"first-page": "699",
"journal-title": "Lancet Respir. Med.",
"key": "B4",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1183/13993003.02226-2020",
"article-title": "Effect of asthma and asthma medication on the prognosis of patients with COVID-19",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "2002226",
"journal-title": "Eur. Respir. J.",
"key": "B5",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03020-3",
"article-title": "Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients",
"author": "Convertino",
"doi-asserted-by": "publisher",
"first-page": "331",
"journal-title": "Crit. Care",
"key": "B6",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1136/thoraxjnl-2021-218629",
"article-title": "Relationship between asthma and severe COVID-19: a national cohort study",
"author": "Dolby",
"doi-asserted-by": "publisher",
"first-page": "120",
"journal-title": "Thorax",
"key": "B7",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.1183/13993003.04451-2020",
"article-title": "Asthma and COVID-19: do we finally have answers?",
"author": "Eger",
"doi-asserted-by": "publisher",
"first-page": "2004451",
"journal-title": "Eur. Respir. J.",
"key": "B8",
"volume": "57",
"year": "2021"
},
{
"author": "Gif",
"key": "B9",
"volume-title": "Global Strategy for asthma management and prevention",
"year": "2022"
},
{
"DOI": "10.1016/j.jaip.2020.11.020",
"article-title": "COVID-19 Susceptibility in Bronchial asthma",
"author": "Green",
"doi-asserted-by": "publisher",
"first-page": "684",
"journal-title": "J. Allergy Clin. Immunol. Pract.",
"key": "B10",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.rmed.2020.106045",
"article-title": "Use of corticosteroids in asthma and COPD patients with or without COVID-19",
"author": "Hasan",
"doi-asserted-by": "publisher",
"first-page": "106045",
"journal-title": "Respir. Med.",
"key": "B11",
"volume": "170",
"year": "2020"
},
{
"DOI": "10.1183/13993003.03142-2020",
"article-title": "The impact of COVID-19 on patients with asthma",
"author": "Izquierdo",
"doi-asserted-by": "publisher",
"first-page": "2003142",
"journal-title": "Eur. Respir. J.",
"key": "B12",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.13063/2327-9214.1244",
"article-title": "A Harmonized data quality assessment Terminology and framework for the Secondary Use of electronic health record data",
"author": "Kahn",
"doi-asserted-by": "publisher",
"first-page": "1244",
"journal-title": "EGEMS (Wash DC)",
"key": "B13",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.4168/aair.2019.11.6.795",
"article-title": "Inhaled corticosteroids in asthma and the risk of pneumonia",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "Allergy Asthma Immunol. Res.",
"key": "B14",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1001/jamanetworkopen.2022.13643",
"article-title": "Analysis of neuropsychiatric diagnoses after montelukast initiation",
"author": "Paljarvi",
"doi-asserted-by": "publisher",
"first-page": "e2213643",
"journal-title": "JAMA Netw. Open",
"key": "B15",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.jaip.2020.06.001",
"article-title": "Impact of COVID-19 on pediatric asthma: practice adjustments and disease burden",
"author": "Papadopoulos",
"doi-asserted-by": "publisher",
"first-page": "2592",
"journal-title": "J. Allergy Clin. Immunol. Pract.",
"key": "B16",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"article-title": "COVID-19-related genes in sputum cells in asthma. relationship to demographic features and corticosteroids",
"author": "Peters",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "Am. J. Respir. Crit. Care Med.",
"key": "B17",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30415-X",
"article-title": "Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform",
"author": "Schultze",
"doi-asserted-by": "publisher",
"first-page": "1106",
"journal-title": "Lancet Respir. Med.",
"key": "B18",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.09.017",
"article-title": "Asthma-associated risk for COVID-19 development",
"author": "Skevaki",
"doi-asserted-by": "publisher",
"first-page": "1295",
"journal-title": "J. Allergy Clin. Immunol.",
"key": "B19",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/S2215-0366(22)00260-7",
"article-title": "Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients",
"author": "Taquet",
"doi-asserted-by": "publisher",
"first-page": "815",
"journal-title": "Lancet Psychiatry",
"key": "B20",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2107058",
"article-title": "Prevention and attenuation of covid-19 with the BNT162b2 and mRNA-1273 vaccines",
"author": "Thompson",
"doi-asserted-by": "publisher",
"first-page": "320",
"journal-title": "N. Engl. J. Med.",
"key": "B21",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"article-title": "Factors associated with COVID-19-related death using OpenSAFELY",
"author": "Williamson",
"doi-asserted-by": "publisher",
"first-page": "430",
"journal-title": "Nature",
"key": "B22",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1007/s15010-018-1229-y",
"article-title": "Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "377",
"journal-title": "Infection",
"key": "B23",
"volume": "47",
"year": "2019"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2023.1204297/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Associations between COVID-19 outcomes and asthmatic patients with inhaled corticosteroid",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}
