Safety evaluation of high bioavailability curcumin in Healthy Japanese Adults: A Randomized, placebo-controlled, double-blind, parallel-group comparison study
et al., Functional Foods in Health and Disease, doi:10.31989/ffhd.v13i12.1207, Dec 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Safety RCTs for highly bioavailable curcumin supplements CR-033P and TS-P1 in healthy Japanese adults, testing 150 mg/day curcumin equivalent of CR-033P or TS-P1 for 12 weeks, and 750 mg/day curcumin equivalent of TS-P1 for 4 weeks. Both trials found no medically concerning changes in liver function markers, hematological parameters, or occurrence of adverse events related to the supplements.
Lee et al., 20 Dec 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 6 authors, study period 25 August, 2022 - 27 November, 2022.
Safety evaluation of high bioavailability curcumin in Healthy Japanese Adults: A Randomized, placebo-controlled, double-blind, parallel-group comparison study
Functional Foods in Health and Disease, doi:10.31989/ffhd.v13i12.1207
Background: Curcumin is the principal component responsible for the pharmacological action of Curcuma longa. It has been proven to exhibit a diverse range of functions. It has been used in many fields as a spice, coloring agent, cosmetic, and food preservative. Objective: To evaluate the safety of the intake of highly bioavailable curcumin (CR-033P and TS-P1) in humans.
Methods: We conducted two trials. The participants were healthy Japanese adults. Participants of tria1 1 (Long-term intake trial) took CR-033P or TS-P1 for 12 weeks (as curcumin 150 mg/day). Participants of Trial 2 (Excessive intake trial) took TS-P1 for 4 weeks (as curcumin 750 mg/day). The safety assessment involved monitoring the occurrence of side effects or adverse events, along with the analysis of urinalysis and blood parameters.
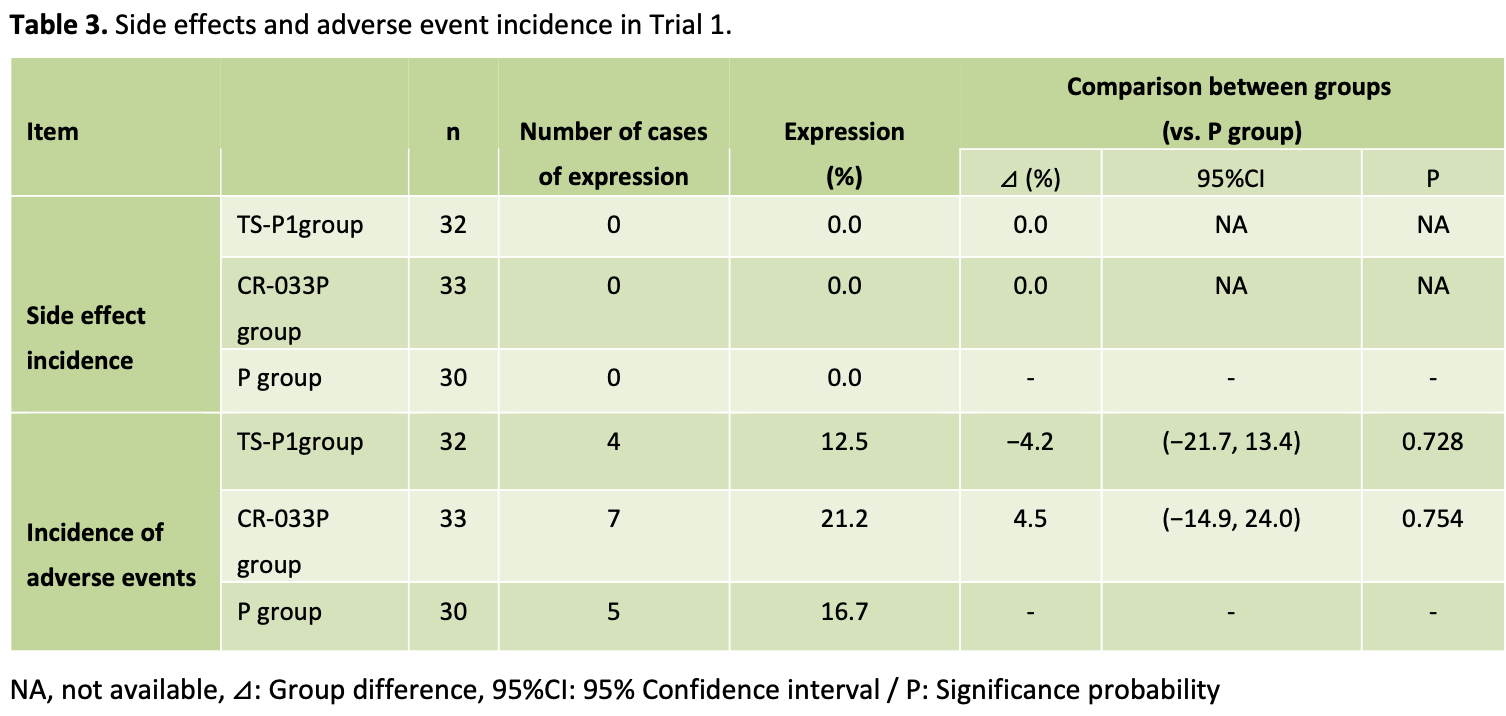
Results: The safety analysis population of Trial 1 included 33 participants in the CR-033P group, 32 participants TS-P1, Functional Foods in Health and Disease 2023;13(12):702-716 FFHD Page 703 of 716 INTRODUCTION Curcuma longa has long garnered significant attention for its pharmacological effects. Not only used as a spice, coloring agent, cosmetic, and food preservative, it has also been utilized for medicinal purposes in India, China, and the rest of South-east Asia [1]. Curcumin, the principal component responsible for the pharmacological action of Curcuma longa, has been proven to exhibit a diverse range of functions. These manifest in hypothermic and hypotensive effects, while also performing roles such as being an anticoagulant, antivenom, antiprotozoal, antifungal, antibacterial, and 30 participants in the Placebo group. The safety analysis population of trial 2 included 22 participants in TS-P1 and 20 participants in the Placebo group. In both Trial 1 and Trial 2, few participants were observed to experience adverse events and however these were not adverse events related to the CR-033P or TS-P1. Results of urinalysis and blood analysis were confirmed to not exhibit medically problematic changes related to the CR-033P or TS-P1. Conclusions: These trials proved the safety of the long-term intake of CR-033P or TS-PI-(as curcumin 150mg/ day) and the safety of the excessive intake of TS-P1 for four weeks (as curcumin 750mg/ day). TS-P1 and CR-033P can be considered a safe curcumin supplement based on these results.
was significantly increased compared to baseline. However, those numbers were changes in the reference range [28] . In a previous study of 8-week intake, there was a significant difference in total bilirubin, Ibil, and triglyceride from placebo group, but the changes were showed to be within the reference range [9] . Based on the results of such previous studies, we further confirmed whether there were any items outside the reference range (Appendix 1, 2). As a result, the item that showed outside the standard value during the test period was the uric acid level of TS-P1 8 weeks after administration, as shown in Appendix 1. The uric acid levels were examined in each subject case and confirmed that no medically problematic changes occurred due to the intake of the test food. Drawing conclusions from Trial 1 and Trial 2 outcomes, it is substantiated that the safety of ingesting 150 mg/day curcumin equivalent of CR-033P or TS-P1 over a 12-week duration, alongside the ingestion of 750 mg/day curcumin equivalent of TS-P1 for 4 weeks, has been confirmed.
CONCLUSION: We investigated the safety of high bioavailability curcumin TS-P1 and CR-033P in healthy Japanese adults. These trials found that the safety of consuming 150 mg/day of curcumin is equivalent to TS-P1 or CR-033P for 12 weeks and 750 mg/day of curcumin equivalent of TS-P1 for 4 weeks. Therefore, the intake of TS-P1 and CR-033P to receive possible health benefits proves to be safe in healthy individuals.
List of..
References
Anand, Kunnumakkara, Newman, Aggarwal, Bioavailability of Curcumin: Problems and Promises, Mol Pharm, doi:10.1021/mp700113r
Chung, Yoon, Cho, Yeo, Shin et al., Comparative pharmacokinetics of Theracurmin, a highly bioavailable curcumin, in healthy adult subjects, Int J Clin Pharmacol Ther, doi:10.5414/CP204058
Cohen, A power primer, Psychol Bull, doi:10.1037/0033-2909.112.1.155
Hirose, Kuwabara, Kanai, Kato, Makino et al., Comparative pharmacokinetics of new curcumin preparations and evidence for increased bioavailability in healthy adult participants, Int J Clin Pharmacol Ther, doi:10.5414/CP204257
Kuwabara, Hirose, Lee, Kakinuma, Baba et al., Effects of Highly Bioavailable Curcumin Supplementation on Common Cold Symptoms and Immune and Inflammatory Functions in Healthy Japanese Subjects: A Randomized Controlled Study, J Diet Suppl, doi:10.1080/19390211.2023.2185723
Lee, Bhatia, Wen, Taira, Lim, Autoimmune hepatitis associated with turmeric consumption, ACG Case Reports J, doi:10.14309/crj.0000000000000320
Lombardi, Crescioli, Maggini, Ippoliti, Ippolito et al., Acute liver injury following turmeric use in Tuscany: an analysis of the Italian Phytovigilance database and systematic review of case reports, Br. J. Clin. Pharmacol, doi:10.1111/bcp.14460
Luber, Rentsch, Lontos, Pope, Aung et al., Turmeric induced liver injury: a report of two cases, Case Reports Hepatol, doi:10.1155/2019/6741213
Martirosyan, Lampert, Ekblad, Classification and regulation of functional food proposed by the Functional Food Center, Functional Food Science, doi:10.31989/ffs.v2i2.890
Mega, Marzi, Kob, Piccin, Floreani, Food and Nutrition in the Pathogenesis of Liver Damage, Nutrients, doi:10.3390/nu13041326
Mhaske, Role of Piperine as an effective bioenhancer in drug absorption, Pharm. Anal. Acta, doi:10.4172/2153-2435.1000591
Ohama, Ikeda, Moriyama, Health foods and food with health claims in Japan, Toxicology, doi:10.1016/B978-0-12-816467-9.00023-X
Pancholi, Smina, Kunnumakkara, Maliakel, Krishnakumar, Safety assessment of a highly bioavailable curcumin-galactomannoside complex (CurQfen) in healthy volunteers, with a special reference to the recent hepatotoxic reports of curcumin supplements: A 90-days prospective study, Toxicology Reports, doi:10.1016/j.toxrep.2021.06.008
Polshettiwar, Sawant, Abhale, Chavan, Baheti et al., Review on Regulation of Herbal Products Used as a Medicine Across the Globe: A Case Study on Turmeric -Golden Medicine, Biomed Pharmacol J, doi:10.13005/bpj/2458
Sharma, Steward, Gescher, Pharmacokinetics and pharmacodynamics of curcumin, doi:10.1007/978-0-387-46401-5_20
Shikishima, Moriyama, Takahashi, Takahashi, Maruyama et al., Novel ELISA technology in assessing undenatured type II collagen in functional foods and dietary supplements used for knee joint health care: its sensitivity, precision, and accuracy, Functional Foods in Health and Disease, doi:10.31989/ffhd.v12i5.933
Shikishima, Yoshinari, Shiojima, Moriyama, Bagchi et al., Safety and toxicological evaluation of a novel Citrus sudachi extract powder, Functional Foods in Health and Disease, doi:10.31989/ffhd.v6i10.301
Shimizu, Newly established regulation in Japan: foods with health claims, Asia Pacific J Clin Nutr, doi:10.1046/j.1440-6047.2002.00007.x
Shiojima, Takahashi, Takahashi, Moriyama, Maruyama et al., Safety of dietary undenatured type II collagen: a pilot open-label overdose clinical investigation, Functional Foods in Health and Disease, doi:10.31989/ffhd.v12i3.897
Stohs, Ray, Issues with human bioavailability determinations of bioactive curcumin. biomed, J. Sci. Tech. Res, doi:10.26717/BJSTR.2019.12.002289
Suhail, Masood, Sharma, John, Dhamoon, Turmeric supplement induced hepatotoxicity: a rare complication of a poorly regulated substance, Clin.Toxicol, doi:10.1080/15563650.2019.1632882
Sunagawa, Hirano, Katanasaka, Miyazaki, Funamoto et al., Colloidal Submicron-Particle Curcumin Exhibits High Absorption Efficiency—A Double-Blind, 3-Way Crossover Study&mdash, J Nutr Sci Vitaminol, doi:10.3177/jnsv.61.37
Suzuki, Baba, Kakinuma, Sano, Tanaka et al., A novel dietary questionnaire: The Calorie and Nutrition Diary (CAND), New Food Ind
Tabanelli, Brogi, Calderone, Improving Curcumin Bioavailability: Current Strategies and Future Perspectives, Pharmaceutics, doi:10.3390/pharmaceutics13101715
Takahashi, Iio, Takara, Safety Evaluation of Theracurmin in Healthy Japanese Adults-A Randomized, Double-blind, Placebo-controlled Parallel-group Study, Jpn Pharmacol Ther
DOI record:
{
"DOI": "10.31989/ffhd.v13i12.1207",
"ISSN": [
"2160-3855",
"2378-7007"
],
"URL": "http://dx.doi.org/10.31989/ffhd.v13i12.1207",
"abstract": "<jats:p>Background: Curcumin is the principal component responsible for the pharmacological action of Curcuma longa. It has been proven to exhibit a diverse range of functions. It has been used in many fields as a spice, coloring agent, cosmetic, and food preservative.Objective: To evaluate the safety of the intake of highly bioavailable curcumin (CR-033P and TS-P1) in humans. Methods: We conducted two trials. The participants were healthy Japanese adults. Participants of tria1 1 (Long-term intake trial) took CR-033P or TS-P1 for 12 weeks (as curcumin 150 mg/day). Participants of Trial 2 (Excessive intake trial) took TS-P1 for 4 weeks (as curcumin 750 mg/day). The safety assessment involved monitoring the occurrence of side effects or adverse events, along with the analysis of urinalysis and blood parameters.Results: The safety analysis population of Trial 1 included 33 participants in the CR-033P group, 32 participants TS-P1, and 30 participants in the Placebo group. The safety analysis population of trial 2 included 22 participants in TS-P1 and 20 participants in the Placebo group. In both Trial 1 and Trial 2, few participants were observed to experience adverse events and however these were not adverse events related to the CR-033P or TS-P1. Results of urinalysis and blood analysis were confirmed to not exhibit medically problematic changes related to the CR-033P or TS-P1.Conclusions: These trials proved the safety of the long-term intake of CR-033P or TS-PI- (as curcumin 150mg/ day) and the safety of the excessive intake of TS- P1 for four weeks (as curcumin 750mg/ day). TS-P1 and CR-033P can be considered a safe curcumin supplement based on these results. Keywords: Curcumin, bioavailability, safety, high dose, long term dose, Healthy Japanese Adults, BMI, Blood pressure Trial registration: Trial 1: UMIN000046160, Trial 2: UMIN000048797. Foundation:Theravalues Corporation</jats:p>",
"author": [
{
"affiliation": [],
"family": "Lee",
"given": "Hyunjin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kuwabara",
"given": "Yoshitaka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirose",
"given": "Akiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kakinuma",
"given": "Toshihiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baba",
"given": "Asami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takara",
"given": "Tsuyoshi",
"sequence": "additional"
}
],
"container-title": "Functional Foods in Health and Disease",
"container-title-short": "FFHD",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
20
]
],
"date-time": "2023-12-20T19:51:06Z",
"timestamp": 1703101866000
},
"deposited": {
"date-parts": [
[
2023,
12,
20
]
],
"date-time": "2023-12-20T19:51:07Z",
"timestamp": 1703101867000
},
"indexed": {
"date-parts": [
[
2023,
12,
21
]
],
"date-time": "2023-12-21T00:25:21Z",
"timestamp": 1703118321128
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12,
20
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2023,
12,
7
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
20
]
],
"date-time": "2023-12-20T00:00:00Z",
"timestamp": 1703030400000
}
}
],
"link": [
{
"URL": "https://www.ffhdj.com/index.php/ffhd/article/viewFile/1207/1991",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.ffhdj.com/index.php/ffhd/article/viewFile/1207/1992",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.ffhdj.com/index.php/ffhd/article/viewFile/1207/1992",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "17268",
"original-title": [],
"prefix": "10.31989",
"published": {
"date-parts": [
[
2023,
12,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
20
]
]
},
"publisher": "Functional Food Center",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.ffhdj.com/index.php/ffhd/article/view/1207"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Nutrition and Dietetics",
"Biochemistry",
"Medicine (miscellaneous)",
"Food Science"
],
"subtitle": [],
"title": "Safety evaluation of high bioavailability curcumin in Healthy Japanese Adults: A Randomized, placebo-controlled, double-blind, parallel-group comparison study",
"type": "journal-article",
"volume": "13"
}
