Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children
et al., JAMA Pediatrics, doi:10.1001/jamapediatrics.2023.2900, Oct 2023
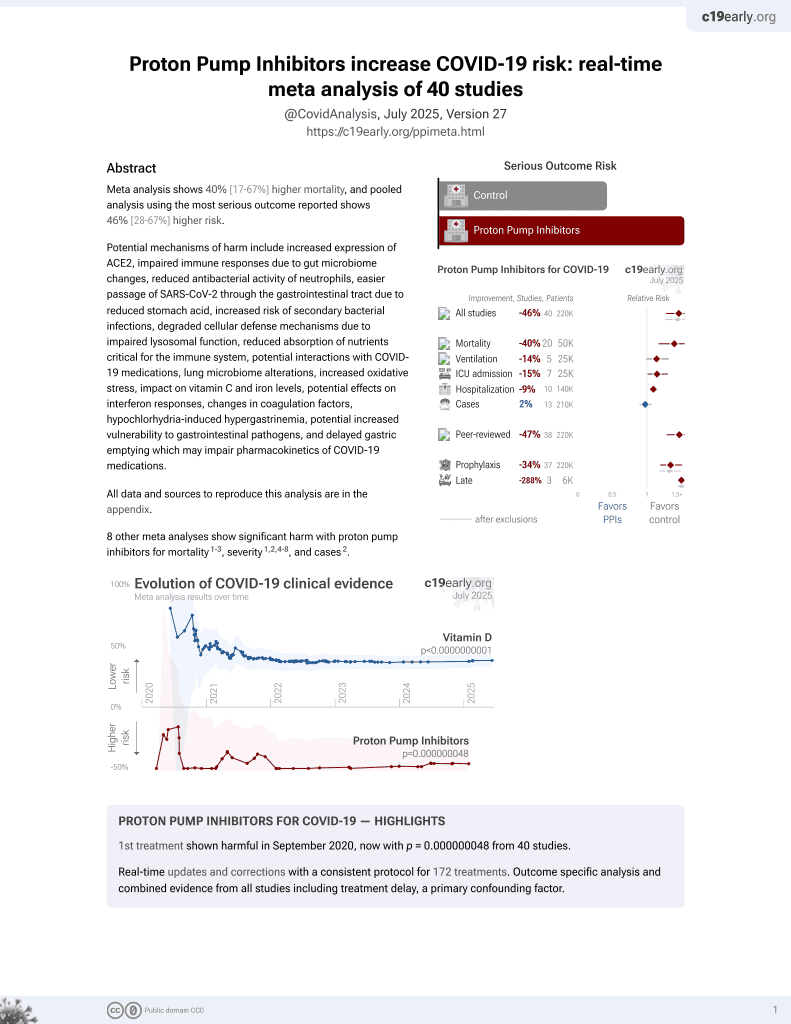
PPIs for COVID-19
1st treatment shown to increase risk in
September 2020, now with p = 0.000000048 from 40 studies.
6,400+ studies for
210+ treatments. c19early.org
|
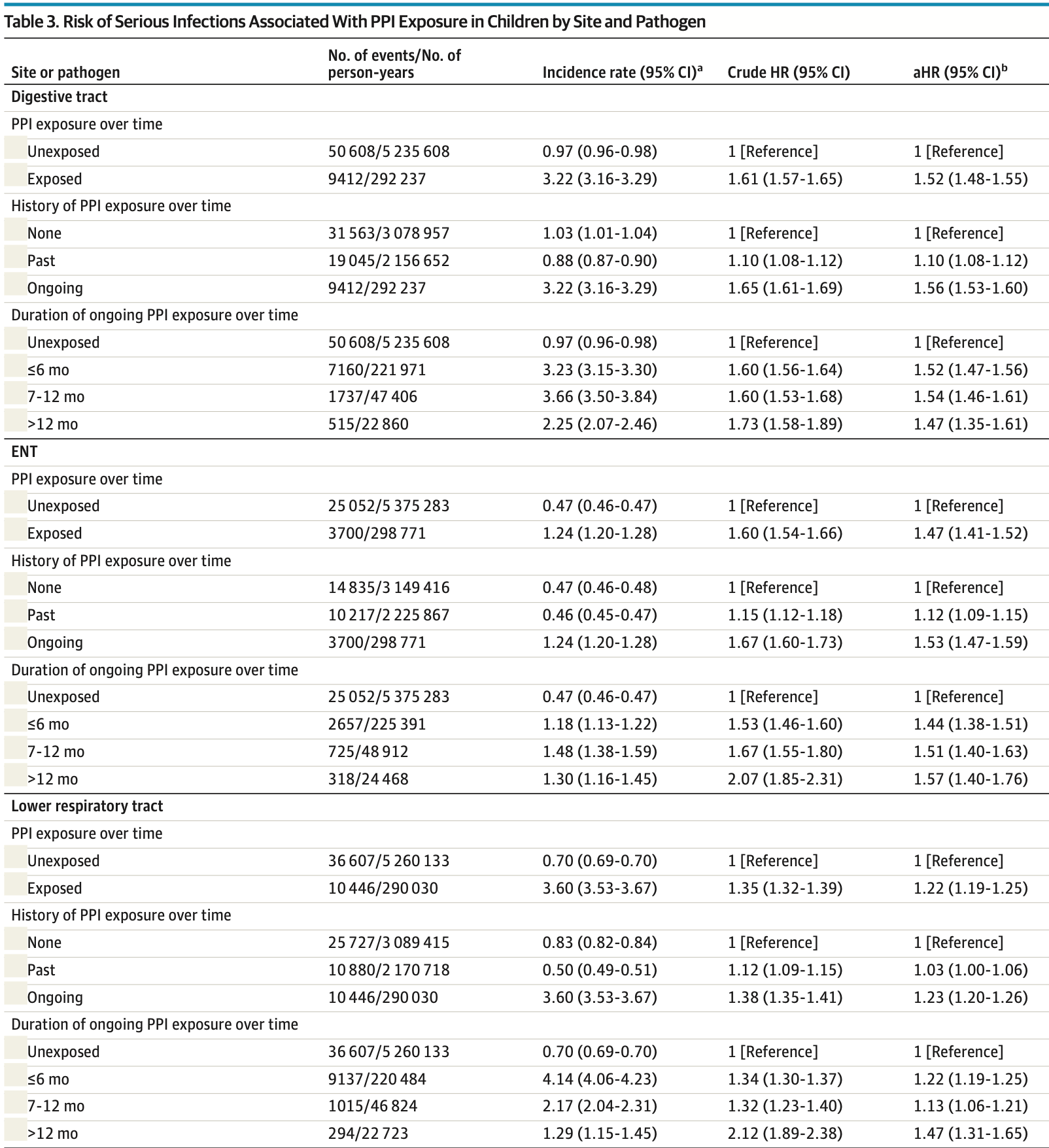
Analysis of 1.2 million children in France showing increased risk of serious infection with proton pump inhibitor (PPI) use. PPI exposure was associated with higher risk of infections in the digestive tract, ear/nose/throat, lower respiratory tract, kidney/urinary tract, and nervous system.
Lassalle et al., 1 Oct 2023, retrospective, France, peer-reviewed, 3 authors.
Contact: lassalle@ansm.sante.fr.
Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children
JAMA Pediatrics, doi:10.1001/jamapediatrics.2023.2900
IMPORTANCE Proton pump inhibitor (PPI) use may lead to infections through alteration of the microbiota or direct action on the immune system. However, only a few studies were conducted in children, with conflicting results. OBJECTIVE To assess the associations between PPI use and serious infections in children, overall and by infection site and pathogen.
DESIGN, SETTING, AND PARTICIPANTS This nationwide cohort study was based on the Mother-Child EPI-MERES Register built from the French Health Data System (SNDS). We included all children born between January 1, 2010, and December 31, 2018, who received a treatment for gastroesophageal reflux disease or other gastric acid-related disorders, namely PPIs, histamine 2 receptor antagonists, or antacids/alginate. The index date was defined as the first date any of these medications was dispensed. Children were followed up until admission to the hospital for serious infection, loss of follow-up, death, or December 31, 2019. EXPOSURE PPI exposure over time. MAIN OUTCOMES AND MEASURES Associations between serious infections and PPI use were estimated by adjusted hazard ratios (aHRs) and 95% CIs using Cox models. PPI use was introduced as time-varying. A 30-day lag was applied to minimize reverse causality. Models were adjusted for sociodemographic data, pregnancy characteristics, child comorbidities, and health care utilization.
RESULTS The study population comprised 1 262 424 children (median [IQR] follow-up, 3.8 [1.8-6.2] years), including 606 645 who received PPI (323 852 male [53.4%]; median [IQR] age at index date, 88 [44-282] days) and 655 779 who did not receive PPI (342 454 male [52.2%]; median [IQR] age, 82 [44-172] days). PPI exposure was associated with an increased risk of serious infections overall (aHR, 1.34; 95% CI, 1.32-1.36). Increased risks were also observed for infections in the digestive tract (aHR, 1.52; 95% CI, 1.48-1.55); ear, nose, and throat sphere (aHR, 1.47; 95% CI, 1.41-1.52); lower respiratory tract (aHR, 1.22; 95% CI, 1.19-1.25); kidneys or urinary tract (aHR, 1.20; 95% CI, 1.15-1.25); and nervous system (aHR, 1.31; 95% CI, 1.11-1.54) and for both bacterial (aHR, 1.56; 95% CI, 1.50-1.63) and viral infections (aHR, 1.30; 95% CI, 1.28-1.33).
CONCLUSIONS AND RELEVANCE In this study, PPI use was associated with increased risks of serious infections in young children. Proton pump inhibitors should not be used without a clear indication in this population.
Conflict of Interest Disclosures: None reported. Data Sharing Statement: See Supplement 2.
References
Anjewierden, Han, Foster, Pant, Deshpande, Risk factors for Clostridium difficile infection in pediatric inpatients: a meta-analysis and systematic review, Infect Control Hosp Epidemiol, doi:10.1017/ice.2019.23
Aznar-Lou, Reilev, Lødrup, Rubio-Valera, Haastrup et al., Use of proton pump inhibitors among Danish children: a 16-year register-based nationwide study, Basic Clin Pharmacol Toxicol, doi:10.1111/bcpt.13191
Blank, Parkin, National study of off-label proton pump inhibitor use among New Zealand infants in the first year of life (2005-2012), J Pediatr Gastroenterol Nutr, doi:10.1097/MPG.0000000000001596
Blank, Parkin, Zeng, Barson, Proton pump inhibitors and infant pneumonia/other lower respiratory tract infections: national nested case-control study, J Pediatr Gastroenterol Nutr, doi:10.1097/MPG.0000000000001984
Budden, Gellatly, Wood, Emerging pathogenic links between microbiota and the gut-lung axis, Nat Rev Microbiol, doi:10.1038/nrmicro.2016.142
Ego, Prunet, Lebreton, Customized and non-customized French intrauterine growth curves: I -Methodology, J Gynecol Obstet Biol Reprod, doi:10.1016/j.jgyn.2015.08.009
Farrell, Lass, Moayyedi, Ward, Thompson, Reduce unnecessary use of proton pump inhibitors, BMJ, doi:10.1136/bmj-2021-069211
Fisher, Fisher, Acid-suppressive therapy and risk of infections: pros and cons, Clin Drug Investig, doi:10.1007/s40261-017-0519-y
Freedberg, Kim, Yang, The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association, Gastroenterology, doi:10.1053/j.gastro.2017.01.031
Gieruszczak-Białek, Konarska, Skórka, Vandenplas, Szajewska, No effect of proton pump inhibitors on crying and irritability in infants: systematic review of randomized controlled trials, J Pediatr, doi:10.1016/j.jpeds.2014.11.030
Haneuse, Vanderweele, Arterburn, Using the E-value to assess the potential effect of unmeasured confounding in observational studies, JAMA, doi:10.1001/jama.2018.21554?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Haut, Pronovost, Surveillance bias in outcomes reporting, JAMA, doi:10.1001/jama.2011.822?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Hung, Teng, Yang, Association between proton pump inhibitor use and CNS infection risk: a retrospective cohort study, J Clin Med, doi:10.3390/jcm7090252
Jena, Sun, Goldman, Confounding in the association of proton pump inhibitor use with risk of community-acquired pneumonia, J Gen Intern Med, doi:10.1007/s11606-012-2211-5
Kyu, Pinho, Wagner, Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 Study, JAMA Pediatr, doi:10.1001/jamapediatrics.2015.4276?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Lassalle, Tri, Bardou, Use of proton pump inhibitors in adults in France: a nationwide drug utilization study, Eur J Clin Pharmacol, doi:10.1007/s00228-019-02810-1
Leung, Hon, Gastroesophageal reflux in children: an updated review, Drugs Context, doi:10.7573/dic.212591
Levy, Hoang, Vandenplas, The effects of proton pump inhibitors on the microbiome in young children, Acta Paediatr, doi:10.1111/apa.15213
Li, Xiong, Yang, Proton pump inhibitors and the risk of hospital-acquired acute kidney injury in children, Ann Transl Med, doi:10.21037/atm-20-2284
Lipsitch, Tchetgen, Cohen, Negative controls: a tool for detecting confounding and bias in observational studies, Epidemiology, doi:10.1097/EDE.0b013e3181d61eeb
Lyamouri, Mårild, Nielsen, Størdal, Proton pump inhibitors for infants in three Scandinavian countries increased from 2007 to 2020 despite international recommendations, Acta Paediatr, doi:10.1111/apa.16491
Malfertheiner, Kandulski, Venerito, Proton-pump inhibitors: understanding the complications and risks, Nat Rev Gastroenterol Hepatol, doi:10.1038/nrgastro.2017.117
Meyer, Drouin, Weill, Carbonnel, Dray-Spira, Pregnancy in women with inflammatory bowel disease: a French nationwide study 2010-2018, Aliment Pharmacol Ther
Meyer, Taine, Drouin, Weill, Carbonnel et al., Serious infections in children born to mothers with inflammatory bowel disease with in utero exposure to thiopurines and anti-tumor necrosis factor, Clin Gastroenterol Hepatol, doi:10.1016/j.cgh.2021.07.028
Miller, Goldacre, Moore, Mode of birth and risk of infection-related hospitalisation in childhood: a population cohort study of 7.17 million births from 4 high-income countries, PLoS Med, doi:10.1371/journal.pmed.1003429
Noh, Jeong, Choi, Prenatal and infant exposure to acid-suppressive medications and risk of allergic diseases in children, JAMA Pediatr, doi:10.1001/jamapediatrics.2022.5193?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Oshima, Wu, Li, Fukui, Watari et al., Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis, J Gastroenterol, doi:10.1007/s00535-017-1369-3
Ronan, Yeasin, Childhood development and the microbiome: the intestinal microbiota in maintenance of health and development of disease during childhood development, Gastroenterology, doi:10.1053/j.gastro.2020.08.065
Rosen, Gastroesophageal reflux in infants: more than just a pHenomenon, JAMA Pediatr, doi:10.1001/jamapediatrics.2013.2911?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Rosen, Vandenplas, Singendonk, Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition, J Pediatr Gastroenterol Nutr, doi:10.1097/MPG.0000000000001889
Sahli, Lapeyre-Mestre, Derumeaux, Moulis, Positive predictive values of selected hospital discharge diagnoses to identify infections responsible for hospitalization in the French national hospital database, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.4006
Schwartz, Hutfless, Herrinton, Proton pump inhibitors, H 2 blocker use, and risk of inflammatory bowel disease in children, J Pediatr Pharmacol Ther, doi:10.5863/1551-6776-24.6.489
Strand, Kim, Peura, 25 Years of proton pump inhibitors: a comprehensive review, Gut Liver, doi:10.5009/gnl15502
Taine, Offredo, Dray-Spira, Weill, Chalumeau et al., Paediatric outpatient prescriptions in France between 2010 and 2019, a nationwide population-based study: paediatric outpatient prescriptions in France, 2010 to 2019, Lancet Reg Health Eur, doi:10.1016/j.lanepe.2021.100129
Tighe, Afzal, Bevan, Hayen, Munro et al., Pharmacological treatment of children with gastro-oesophageal reflux, Cochrane Database Syst Rev, doi:10.1002/14651858.CD008550.pub2
Tuppin, Rivière, Deutsch, Gastaldi-Menager, Sabaté, Burden of drug use for gastrointestinal symptoms and functional gastrointestinal disorders in France: a national study using reimbursement data for 57 million inhabitants, Therap Adv Gastroenterol, doi:10.1177/1756284819853790
Van Der Sande, Jöbsis, Bannier, The risk of community-acquired pneumonia in children using gastric acid suppressants, Eur Respir J, doi:10.1183/13993003.03229-2020
Vanderweele, Ding, Sensitivity analysis in observational research: introducing the E-value, Ann Intern Med, doi:10.7326/M16-2607
Wang, Bell, Tan, Thomas, Dooley et al., Proton pump inhibitors and infection-related hospitalizations among residents of long-term care facilities: a case-control study, Drugs Aging, doi:10.1007/s40266-019-00704-6
Wang, Svanström, Wintzell, Ludvigsson, Pasternak, Association between proton pump inhibitor use and risk of pneumonia in children: nationwide self-controlled case series study in Sweden, BMJ Open, doi:10.1136/bmjopen-2022-060771
Wang, Wintzell, Ludvigsson, Svanström, Pasternak, Association between proton pump inhibitor use and risk of asthma in children, JAMA Pediatr, doi:10.1001/jamapediatrics.2020.5710?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Wang, Wintzell, Ludvigsson, Svanström, Pasternak, Association between proton pump inhibitor use and risk of fracture in children, JAMA Pediatr, doi:10.1001/jamapediatrics.2020.0007?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamapediatrics.2023.2900
Yang, Trinh, Chalumeau, Pediatric prescriptions of proton pump inhibitors in France (2009-2019): a time-series analysis of trends and practice guidelines impact, J Pediatr, doi:10.1016/j.jpeds.2022.01.041
DOI record:
{
"DOI": "10.1001/jamapediatrics.2023.2900",
"ISSN": [
"2168-6203"
],
"URL": "http://dx.doi.org/10.1001/jamapediatrics.2023.2900",
"abstract": "<jats:sec id=\"ab-poi230045-4\"><jats:title>Importance</jats:title><jats:p>Proton pump inhibitor (PPI) use may lead to infections through alteration of the microbiota or direct action on the immune system. However, only a few studies were conducted in children, with conflicting results.</jats:p></jats:sec><jats:sec id=\"ab-poi230045-5\"><jats:title>Objective</jats:title><jats:p>To assess the associations between PPI use and serious infections in children, overall and by infection site and pathogen.</jats:p></jats:sec><jats:sec id=\"ab-poi230045-6\"><jats:title>Design, Setting, and Participants</jats:title><jats:p>This nationwide cohort study was based on the Mother-Child EPI-MERES Register built from the French Health Data System (SNDS). We included all children born between January 1, 2010, and December 31, 2018, who received a treatment for gastroesophageal reflux disease or other gastric acid–related disorders, namely PPIs, histamine 2 receptor antagonists, or antacids/alginate. The index date was defined as the first date any of these medications was dispensed. Children were followed up until admission to the hospital for serious infection, loss of follow-up, death, or December 31, 2019.</jats:p></jats:sec><jats:sec id=\"ab-poi230045-7\"><jats:title>Exposure</jats:title><jats:p>PPI exposure over time.</jats:p></jats:sec><jats:sec id=\"ab-poi230045-8\"><jats:title>Main Outcomes and Measures</jats:title><jats:p>Associations between serious infections and PPI use were estimated by adjusted hazard ratios (aHRs) and 95% CIs using Cox models. PPI use was introduced as time-varying. A 30-day lag was applied to minimize reverse causality. Models were adjusted for sociodemographic data, pregnancy characteristics, child comorbidities, and health care utilization.</jats:p></jats:sec><jats:sec id=\"ab-poi230045-9\"><jats:title>Results</jats:title><jats:p>The study population comprised 1 262 424 children (median [IQR] follow-up, 3.8 [1.8-6.2] years), including 606 645 who received PPI (323 852 male [53.4%]; median [IQR] age at index date, 88 [44-282] days) and 655 779 who did not receive PPI (342 454 male [52.2%]; median [IQR] age, 82 [44-172] days). PPI exposure was associated with an increased risk of serious infections overall (aHR, 1.34; 95% CI, 1.32-1.36). Increased risks were also observed for infections in the digestive tract (aHR, 1.52; 95% CI, 1.48-1.55); ear, nose, and throat sphere (aHR, 1.47; 95% CI, 1.41-1.52); lower respiratory tract (aHR, 1.22; 95% CI, 1.19-1.25); kidneys or urinary tract (aHR, 1.20; 95% CI, 1.15-1.25); and nervous system (aHR, 1.31; 95% CI, 1.11-1.54) and for both bacterial (aHR, 1.56; 95% CI, 1.50-1.63) and viral infections (aHR, 1.30; 95% CI, 1.28-1.33).</jats:p></jats:sec><jats:sec id=\"ab-poi230045-10\"><jats:title>Conclusions and Relevance</jats:title><jats:p>In this study, PPI use was associated with increased risks of serious infections in young children. Proton pump inhibitors should not be used without a clear indication in this population.</jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "EPI-PHARE, Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], and French National Health Insurance [CNAM]), Saint-Denis, France"
}
],
"family": "Lassalle",
"given": "Marion",
"sequence": "first"
},
{
"affiliation": [
{
"name": "EPI-PHARE, Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], and French National Health Insurance [CNAM]), Saint-Denis, France"
},
{
"name": "Versailles Saint-Quentin-en-Yvelines University, Versailles, France"
}
],
"family": "Zureik",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EPI-PHARE, Epidemiology of Health Products (French National Agency for the Safety of Medicines and Health Products [ANSM], and French National Health Insurance [CNAM]), Saint-Denis, France"
}
],
"family": "Dray-Spira",
"given": "Rosemary",
"sequence": "additional"
}
],
"container-title": "JAMA Pediatrics",
"container-title-short": "JAMA Pediatr",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
14
]
],
"date-time": "2023-08-14T15:01:13Z",
"timestamp": 1692025273000
},
"deposited": {
"date-parts": [
[
2023,
10,
2
]
],
"date-time": "2023-10-02T15:02:59Z",
"timestamp": 1696258979000
},
"indexed": {
"date-parts": [
[
2024,
9,
23
]
],
"date-time": "2024-09-23T06:43:38Z",
"timestamp": 1727073818939
},
"is-referenced-by-count": 23,
"issue": "10",
"issued": {
"date-parts": [
[
2023,
10,
1
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2023,
10,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamapediatrics/articlepdf/2808367/jamapediatrics_lassalle_2023_oi_230045_1695847051.54709.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1028",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2023,
10,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
10,
1
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1097/MPG.0000000000001889",
"article-title": "Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.",
"author": "Rosen",
"doi-asserted-by": "publisher",
"first-page": "516",
"issue": "3",
"journal-title": "J Pediatr Gastroenterol Nutr",
"key": "poi230045r1",
"volume": "66",
"year": "2018"
},
{
"DOI": "10.1001/jamapediatrics.2013.2911",
"article-title": "Gastroesophageal reflux in infants: more than just a pHenomenon.",
"author": "Rosen",
"doi-asserted-by": "publisher",
"first-page": "83",
"issue": "1",
"journal-title": "JAMA Pediatr",
"key": "poi230045r2",
"volume": "168",
"year": "2014"
},
{
"DOI": "10.7573/17404398",
"article-title": "Gastroesophageal reflux in children: an updated review.",
"author": "Leung",
"doi-asserted-by": "publisher",
"journal-title": "Drugs Context",
"key": "poi230045r3",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1016/j.lanepe.2021.100129",
"article-title": "Paediatric outpatient prescriptions in France between 2010 and 2019, a nationwide population-based study: paediatric outpatient prescriptions in France, 2010 to 2019.",
"author": "Taine",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Reg Health Eur",
"key": "poi230045r4",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1097/MPG.0000000000001596",
"article-title": "National study of off-label proton pump inhibitor use among New Zealand infants in the first year of life (2005-2012).",
"author": "Blank",
"doi-asserted-by": "publisher",
"first-page": "179",
"issue": "2",
"journal-title": "J Pediatr Gastroenterol Nutr",
"key": "poi230045r5",
"volume": "65",
"year": "2017"
},
{
"DOI": "10.1111/bcpt.2019.124.issue-6",
"article-title": "Use of proton pump inhibitors among Danish children: a 16-year register-based nationwide study.",
"author": "Aznar-Lou",
"doi-asserted-by": "publisher",
"first-page": "704",
"issue": "6",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "poi230045r6",
"volume": "124",
"year": "2019"
},
{
"DOI": "10.1111/apa.v111.11",
"article-title": "Proton pump inhibitors for infants in three Scandinavian countries increased from 2007 to 2020 despite international recommendations.",
"author": "Lyamouri",
"doi-asserted-by": "publisher",
"first-page": "2222",
"issue": "11",
"journal-title": "Acta Paediatr",
"key": "poi230045r7",
"volume": "111",
"year": "2022"
},
{
"DOI": "10.1001/jamapediatrics.2020.0007",
"article-title": "Association between proton pump inhibitor use and risk of fracture in children.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "543",
"issue": "6",
"journal-title": "JAMA Pediatr",
"key": "poi230045r8",
"volume": "174",
"year": "2020"
},
{
"DOI": "10.21037/atm",
"article-title": "Proton pump inhibitors and the risk of hospital-acquired acute kidney injury in children.",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "1438",
"issue": "21",
"journal-title": "Ann Transl Med",
"key": "poi230045r9",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1001/jamapediatrics.2022.5193",
"article-title": "Prenatal and infant exposure to acid-suppressive medications and risk of allergic diseases in children.",
"author": "Noh",
"doi-asserted-by": "publisher",
"first-page": "267",
"issue": "3",
"journal-title": "JAMA Pediatr",
"key": "poi230045r10",
"volume": "177",
"year": "2023"
},
{
"DOI": "10.1001/jamapediatrics.2020.5710",
"article-title": "Association between proton pump inhibitor use and risk of asthma in children.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "394",
"issue": "4",
"journal-title": "JAMA Pediatr",
"key": "poi230045r11",
"volume": "175",
"year": "2021"
},
{
"DOI": "10.5863/1551-6776-24.6.489",
"article-title": "Proton pump inhibitors, H2 blocker use, and risk of inflammatory bowel disease in children.",
"author": "Schwartz",
"doi-asserted-by": "publisher",
"first-page": "489",
"issue": "6",
"journal-title": "J Pediatr Pharmacol Ther",
"key": "poi230045r12",
"volume": "24",
"year": "2019"
},
{
"DOI": "10.1038/nrgastro.2017.117",
"article-title": "Proton-pump inhibitors: understanding the complications and risks.",
"author": "Malfertheiner",
"doi-asserted-by": "publisher",
"first-page": "697",
"issue": "12",
"journal-title": "Nat Rev Gastroenterol Hepatol",
"key": "poi230045r13",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1007/s40261-017-0519-y",
"article-title": "Acid-suppressive therapy and risk of infections: pros and cons.",
"author": "Fisher",
"doi-asserted-by": "publisher",
"first-page": "587",
"issue": "7",
"journal-title": "Clin Drug Investig",
"key": "poi230045r14",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1001/jamapediatrics.2015.4276",
"article-title": "Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 Study.",
"author": "Kyu",
"doi-asserted-by": "publisher",
"first-page": "267",
"issue": "3",
"journal-title": "JAMA Pediatr",
"key": "poi230045r15",
"volume": "170",
"year": "2016"
},
{
"DOI": "10.1016/j.cgh.2021.07.028",
"article-title": "Serious infections in children born to mothers with inflammatory bowel disease with in utero exposure to thiopurines and anti-tumor necrosis factor.",
"author": "Meyer",
"doi-asserted-by": "publisher",
"first-page": "1269",
"issue": "6",
"journal-title": "Clin Gastroenterol Hepatol",
"key": "poi230045r16",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1111/apt.v52.9",
"article-title": "Pregnancy in women with inflammatory bowel disease: a French nationwide study 2010-2018.",
"author": "Meyer",
"doi-asserted-by": "publisher",
"first-page": "1480",
"issue": "9",
"journal-title": "Aliment Pharmacol Ther",
"key": "poi230045r17",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.5009/gnl15502",
"article-title": "25 Years of proton pump inhibitors: a comprehensive review.",
"author": "Strand",
"doi-asserted-by": "publisher",
"first-page": "27",
"issue": "1",
"journal-title": "Gut Liver",
"key": "poi230045r18",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1136/bmj-2021-069211",
"article-title": "Reduce unnecessary use of proton pump inhibitors.",
"author": "Farrell",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "poi230045r19",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1136/bmjopen-2022-060771",
"article-title": "Association between proton pump inhibitor use and risk of pneumonia in children: nationwide self-controlled case series study in Sweden.",
"author": "Wang",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "BMJ Open",
"key": "poi230045r20",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1016/j.jgyn.2015.08.009",
"article-title": "Customized and non-customized French intrauterine growth curves: I - Methodology [in French].",
"author": "Ego",
"doi-asserted-by": "publisher",
"first-page": "155",
"issue": "2",
"journal-title": "J Gynecol Obstet Biol Reprod (Paris)",
"key": "poi230045r21",
"volume": "45",
"year": "2016"
},
{
"DOI": "10.1016/j.jpeds.2022.01.041",
"article-title": "Pediatric prescriptions of proton pump inhibitors in France (2009-2019): a time-series analysis of trends and practice guidelines impact.",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "158",
"journal-title": "J Pediatr",
"key": "poi230045r22",
"volume": "245",
"year": "2022"
},
{
"DOI": "10.1177/1756284819853790",
"article-title": "Burden of drug use for gastrointestinal symptoms and functional gastrointestinal disorders in France: a national study using reimbursement data for 57 million inhabitants.",
"author": "Tuppin",
"doi-asserted-by": "publisher",
"journal-title": "Therap Adv Gastroenterol",
"key": "poi230045r23",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1001/jama.2018.21554",
"article-title": "Using the E-value to assess the potential effect of unmeasured confounding in observational studies.",
"author": "Haneuse",
"doi-asserted-by": "publisher",
"first-page": "602",
"issue": "6",
"journal-title": "JAMA",
"key": "poi230045r24",
"volume": "321",
"year": "2019"
},
{
"DOI": "10.7326/M16-2607",
"article-title": "Sensitivity analysis in observational research: introducing the E-value.",
"author": "VanderWeele",
"doi-asserted-by": "publisher",
"first-page": "268",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "poi230045r25",
"volume": "167",
"year": "2017"
},
{
"DOI": "10.1097/EDE.0b013e3181d61eeb",
"article-title": "Negative controls: a tool for detecting confounding and bias in observational studies.",
"author": "Lipsitch",
"doi-asserted-by": "publisher",
"first-page": "383",
"issue": "3",
"journal-title": "Epidemiology",
"key": "poi230045r26",
"volume": "21",
"year": "2010"
},
{
"DOI": "10.1111/apa.v109.8",
"article-title": "The effects of proton pump inhibitors on the microbiome in young children.",
"author": "Levy",
"doi-asserted-by": "publisher",
"first-page": "1531",
"issue": "8",
"journal-title": "Acta Paediatr",
"key": "poi230045r27",
"volume": "109",
"year": "2020"
},
{
"DOI": "10.1053/j.gastro.2020.08.065",
"article-title": "Childhood development and the microbiome: the intestinal microbiota in maintenance of health and development of disease during childhood development.",
"author": "Ronan",
"doi-asserted-by": "publisher",
"first-page": "495",
"issue": "2",
"journal-title": "Gastroenterology",
"key": "poi230045r28",
"volume": "160",
"year": "2021"
},
{
"DOI": "10.1053/j.gastro.2017.01.031",
"article-title": "The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association.",
"author": "Freedberg",
"doi-asserted-by": "publisher",
"first-page": "706",
"issue": "4",
"journal-title": "Gastroenterology",
"key": "poi230045r29",
"volume": "152",
"year": "2017"
},
{
"DOI": "10.1038/nrmicro.2016.142",
"article-title": "Emerging pathogenic links between microbiota and the gut-lung axis.",
"author": "Budden",
"doi-asserted-by": "publisher",
"first-page": "55",
"issue": "1",
"journal-title": "Nat Rev Microbiol",
"key": "poi230045r30",
"volume": "15",
"year": "2017"
},
{
"DOI": "10.1017/ice.2019.23",
"article-title": "Risk factors for Clostridium difficile infection in pediatric inpatients: a meta-analysis and systematic review.",
"author": "Anjewierden",
"doi-asserted-by": "publisher",
"first-page": "420",
"issue": "4",
"journal-title": "Infect Control Hosp Epidemiol",
"key": "poi230045r31",
"volume": "40",
"year": "2019"
},
{
"DOI": "10.1007/s00535-017-1369-3",
"article-title": "Magnitude and direction of the association between Clostridium difficile infection and proton pump inhibitors in adults and pediatric patients: a systematic review and meta-analysis.",
"author": "Oshima",
"doi-asserted-by": "publisher",
"first-page": "84",
"issue": "1",
"journal-title": "J Gastroenterol",
"key": "poi230045r32",
"volume": "53",
"year": "2018"
},
{
"DOI": "10.1183/13993003.03229-2020",
"article-title": "The risk of community-acquired pneumonia in children using gastric acid suppressants.",
"author": "van der Sande",
"doi-asserted-by": "publisher",
"issue": "4",
"journal-title": "Eur Respir J",
"key": "poi230045r33",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1097/MPG.0000000000001984",
"article-title": "Proton pump inhibitors and infant pneumonia/other lower respiratory tract infections: national nested case-control study.",
"author": "Blank",
"doi-asserted-by": "publisher",
"first-page": "335",
"issue": "3",
"journal-title": "J Pediatr Gastroenterol Nutr",
"key": "poi230045r34",
"volume": "67",
"year": "2018"
},
{
"DOI": "10.3390/jcm7090252",
"article-title": "Association between proton pump inhibitor use and CNS infection risk: a retrospective cohort study.",
"author": "Hung",
"doi-asserted-by": "publisher",
"issue": "9",
"journal-title": "J Clin Med",
"key": "poi230045r35",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1007/s40266-019-00704-6",
"article-title": "Proton pump inhibitors and infection-related hospitalizations among residents of long-term care facilities: a case-control study.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1027",
"issue": "11",
"journal-title": "Drugs Aging",
"key": "poi230045r36",
"volume": "36",
"year": "2019"
},
{
"DOI": "10.1007/s11606-012-2211-5",
"article-title": "Confounding in the association of proton pump inhibitor use with risk of community-acquired pneumonia.",
"author": "Jena",
"doi-asserted-by": "publisher",
"first-page": "223",
"issue": "2",
"journal-title": "J Gen Intern Med",
"key": "poi230045r37",
"volume": "28",
"year": "2013"
},
{
"DOI": "10.1002/14651858.CD008550.pub2",
"article-title": "Pharmacological treatment of children with gastro-oesophageal reflux.",
"author": "Tighe",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "Cochrane Database Syst Rev",
"key": "poi230045r38",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1002/pds.v25.7",
"article-title": "Positive predictive values of selected hospital discharge diagnoses to identify infections responsible for hospitalization in the French national hospital database.",
"author": "Sahli",
"doi-asserted-by": "publisher",
"first-page": "785",
"issue": "7",
"journal-title": "Pharmacoepidemiol Drug Saf",
"key": "poi230045r39",
"volume": "25",
"year": "2016"
},
{
"DOI": "10.1001/jama.2011.822",
"article-title": "Surveillance bias in outcomes reporting.",
"author": "Haut",
"doi-asserted-by": "publisher",
"first-page": "2462",
"issue": "23",
"journal-title": "JAMA",
"key": "poi230045r40",
"volume": "305",
"year": "2011"
},
{
"DOI": "10.1016/j.jpeds.2014.11.030",
"article-title": "No effect of proton pump inhibitors on crying and irritability in infants: systematic review of randomized controlled trials.",
"author": "Gieruszczak-Bialek",
"doi-asserted-by": "publisher",
"first-page": "767",
"issue": "3",
"journal-title": "J Pediatr",
"key": "poi230045r41",
"volume": "166",
"year": "2015"
},
{
"DOI": "10.1371/journal.pmed.1003429",
"article-title": "Mode of birth and risk of infection-related hospitalisation in childhood: a population cohort study of 7.17 million births from 4 high-income countries.",
"author": "Miller",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "PLoS Med",
"key": "poi230045r42",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s00228-019-02810-1",
"article-title": "Use of proton pump inhibitors in adults in France: a nationwide drug utilization study.",
"author": "Lassalle",
"doi-asserted-by": "publisher",
"first-page": "449",
"issue": "3",
"journal-title": "Eur J Clin Pharmacol",
"key": "poi230045r43",
"volume": "76",
"year": "2020"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamapediatrics/fullarticle/2808367"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Proton Pump Inhibitor Use and Risk of Serious Infections in Young Children",
"type": "journal-article",
"volume": "177"
}