Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis
et al., Journal of Thoracic Disease, doi:10.21037/jtd-23-1604, PROSPERO CRD42023475640, Jun 2024
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
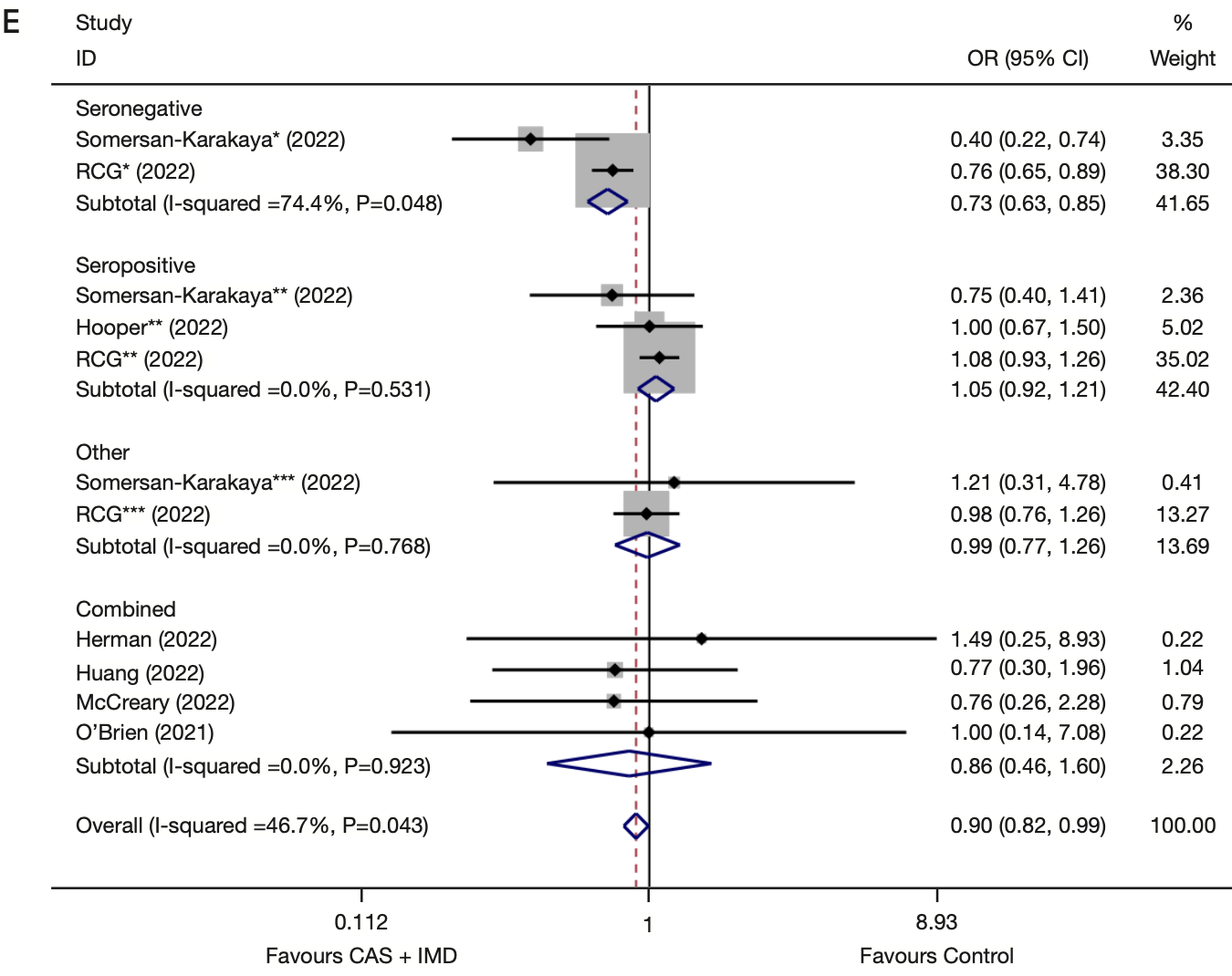
Meta analysis of 12 RCTs with 27,179 participants showing that casirivimab/imdevimab treatment significantly reduced viral load, all-cause mortality, and cases. Efficacy was better in patients who were seronegative at baseline.
Currently there are 34 casirivimab/imdevimab studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 19% lower [-18‑45%] |
| Ventilation | 0% higher [-11‑13%] |
| ICU admission | 35% lower [-8‑60%] |
| Hospitalization | 39% lower [16‑55%] |
| Cases | 72% fewer [32‑89%] |
Cui et al., 30 Jun 2024, USA, peer-reviewed, 6 authors, trial PROSPERO CRD42023475640.
Contact: zhangyezy1986@163.com, 00135116@zjxu.edu.cn.
Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis
Journal of Thoracic Disease, doi:10.21037/jtd-23-1604
Background: The ongoing global epidemic of coronavirus disease 2019 (COVID-19) has created a serious public health problem. The selection of safe and effective therapeutic agents is of paramount importance. This systematic review aims to evaluate the efficacy and safety of the combination of casirivimab and imdevimab in the treatment of global cases of COVID-19. Methods: To identify randomized controlled trials (RCTs) investigating the combined administration of casirivimab and imdevimab for COVID-19 management, a comprehensive search was conducted across multiple databases including PubMed, Web of Science, Embase, and the Cochrane Library from their inception to September 10, 2022. Data on the efficacy and safety of casirivimab and imdevimab were extracted. Subgroup analyses and sensitivity analyses were performed. Results: A total of 851 articles were searched. Twelve studies were finally included in the meta-analysis, with 27,179 participants. Dichotomous and continuous variables were presented as odds ratios (ORs) and weighted mean differences (WMDs) with their 95% confidence intervals (CIs), respectively. Compared to placebo or alternative medications, the combination of casirivimab and imdevimab reduced viral load (WMD: -0.73, 95% CI: -1.09 to -0.38, P<0.01), all-cause mortality (OR =0.90, 95% CI: 0.82-0.99, P=0.03), the incidence of any serious adverse events (OR =0.80, 95% CI: 0.67-0.95, P=0.01), the incidence of Grade 3 or more severe adverse events (OR =0.76, 95% CI: 0.62-0.92, P=0.01), the likelihood of contracting COVID-19, the incidence of hospitalization, emergency room visits, and mortality (OR =0.54, 95% CI: 0.32-0.93, P=0.03).
Conclusions: The monoclonal antibody combination of casirivimab and imdevimab is effective in treating patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as they can reduce viral load, all-cause mortality, infection rates, and the incidence of clinical outcomes of special interest after treatment, while maintaining a favorable safety profile.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups. com/article/view/10.21037/jtd-23-1604/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the noncommercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Bierle, Ganesh, Razonable, Breakthrough COVID-19 and casirivimab-imdevimab treatment during a SARS-CoV-2 B1.617.2 (Delta) surge, J Clin Virol
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med
Cicchitto, Cardillo, De Martinis, Effects of Casirivimab/Imdevimab Monoclonal Antibody Treatment among Vaccinated Patients Infected by SARS-CoV-2 Delta Variant, Viruses
Clark, Jit, Gash, Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study, Lancet Glob Health
Deng, Heybati, Ramaraju, Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: a systematic review and network meta-analysis, Infection
Deng, Zhou, Ali, Efficacy and safety of ivermectin for the treatment of COVID-19: a systematic review and meta-analysis, QJM
Deng, Zhou, Hou, Efficacy of lopinavirritonavir combination therapy for the treatment of hospitalized COVID-19 patients: a meta-analysis, Future Virol
Gao, Ao, Hao, Casirivimab-imdevimab treatment is associated with reduced rates of mortality and hospitalization in patients with COVID-19: A systematic review with meta-analysis, J Infect
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, JAMA
Gungor, Nematollahi, Effectiveness of Casirivimab-Imdevimab Monoclonal Antibody Treatment Among High-Risk Patients With Severe Acute Respiratory Syndrome Coronavirus 2 B.1.617.2 (Delta Variant) Infection, Open Forum Infect Dis
Hegazy, Tharwat, Hassan, Clinical study to compare the efficacy and safety of casirivimab & imdevimab, remdesivir, and favipravir in hospitalized COVID-19 patients, J Clin Virol Plus
Hegazy, Tharwat, Hassan, Comparing efficacy of regen-cov, remdesivir, and favipiravir in reducing invasive mechanical ventilation need in hospitalized COVID-19 patients, World J Clin Cases
Hegazy, Tharwat, Hassan, Study to compare the effect of casirivimab and imdevimab, remdesivir, and favipiravir on progression and multi-organ function of hospitalized COVID-19 patients, Open Med (Wars)
Herman, Brien, Forleo-Neto, Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: a randomised, double-blind, placebo-controlled trial, Lancet Infect Dis
Higgins, Altman, Gøtzsche, The Cochrane Collaboration's tool for assessing risk of bias in randomised trials, BMJ
Hooper, Somersan-Karakaya, Mccarthy, Casirivimab and Imdevimab Treatment Reduces Viral Load and Improves Clinical Outcomes in Seropositive Hospitalized COVID-19 Patients with Nonneutralizing or Borderline Neutralizing Antibodies, mBio
Huang, Mccreary, Bariola, Effectiveness of Casirivimab-Imdevimab and Sotrovimab During a SARS-CoV-2 Delta Variant Surge: A Cohort Study and Randomized Comparative Effectiveness Trial, JAMA Netw Open
Hussein, Wei, Mastey, Real-world effectiveness of casirivimab and imdevimab among patients diagnosed with COVID-19 in the ambulatory setting: a retrospective cohort study using a large claims database, BMJ Open
Isa, Forleo-Neto, Meyer, Repeat subcutaneous administration of casirivimab and imdevimab in adults is well-tolerated and prevents the occurrence of COVID-19, Int J Infect Dis
Jalbert, Hussein, Mastey, Effectiveness of Subcutaneous Casirivimab and Imdevimab in Ambulatory Patients with COVID-19, Infect Dis Ther
Ko, Danielson, Town, Risk Factors for Coronavirus Disease 2019 (COVID-19)-Associated Hospitalization: COVID-19-Associated Hospitalization Surveillance Network and Behavioral Risk Factor Surveillance System, Clin Infect Dis
Kreuzberger, Hirsch, Chai, SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19, Cochrane Database Syst Rev
Lin, Hung, Lai, The impact of neutralizing monoclonal antibodies on the outcomes of COVID-19 outpatients: A systematic review and metaanalysis of randomized controlled trials, J Med Virol
Liu, Wang, Nair, Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike, Nature
Mazzaferri, Mirandola, Savoldi, Exploratory data on the clinical efficacy of monoclonal antibodies against SARS-CoV-2 Omicron variant of concern, Elife
Mccreary, Bariola, Minnier, The comparative effectiveness of COVID-19 monoclonal antibodies: A learning health system randomized clinical trial, Contemp Clin Trials
Meo, Meo, Ff, Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics, Eur Rev Med Pharmacol Sci
Norton, Ali, Sivapalasingam, REGEN-COV Antibody Combination in Outpatients With COVID-19 -Phase 1/2 Results, medRxiv, doi:10.1101/2021.06.09.21257915
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19, N Engl J Med
O'brien, Forleo-Neto, Sarkar, Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection: A Randomized Clinical Trial, JAMA
Portal-Celhay, Forleo-Neto, Eagan, Phase 2 dose-ranging study of the virologic efficacy and safety of the combination COVID-19 antibodies casirivimab and imdevimab in the outpatient setting, medRxiv, doi:10.1101/2021.11.09.21265912
Portal-Celhay, Forleo-Neto, Eagan, Virologic Efficacy of Casirivimab and Imdevimab COVID-19 Antibody Combination in Outpatients With SARS-CoV-2 Infection: A Phase 2 Dose-Ranging Randomized Clinical Trial, JAMA Netw Open
Siemieniuk, Bartoszko, Martinez, Antibody and cellular therapies for treatment of covid-19: a living systematic review and network meta-analysis, BMJ
Somersan-Karakaya, Mylonakis, Menon, Casirivimab and Imdevimab for the Treatment of Hospitalized Patients With COVID-19, J Infect Dis
Stein, Oviedo-Orta, Kampman, Compassionate Use of REGEN-COV® in Patients With Coronavirus Disease 2019 (COVID-19) and © Journal of Thoracic Disease. All rights reserved, Clin Infect Dis, doi:10.21037/jtd-23-1604Immunodeficiency-Associated
Tao, Tzou, Pond, Susceptibility of SARS-CoV-2 Omicron Variants to Therapeutic Monoclonal Antibodies: Systematic Review and Metaanalysis, Microbiol Spectr
Trougakos, Terpos, Alexopoulos, Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis, Trends Mol Med
Walls, Park, Tortorici, Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein, Cell
Wang, Li, Drabek, A human monoclonal antibody blocking SARS-CoV-2 infection, Nat Commun
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N Engl J Med
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med
Weisblum, Schmidt, Zhang, Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants, Elife
Yan, Yang, Lai, COVID-19 Vaccinations: A Comprehensive Review of Their Safety and Efficacy in Special Populations, Vaccines
Şimşek-Yavuz, Çelikyurt, An update of anti-viral treatment of COVID-19, Turk J Med Sci
DOI record:
{
"DOI": "10.21037/jtd-23-1604",
"ISSN": [
"2072-1439",
"2077-6624"
],
"URL": "http://dx.doi.org/10.21037/jtd-23-1604",
"author": [
{
"affiliation": [],
"family": "Cui",
"given": "Zhifang",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Hongwu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zou",
"given": "Heng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Lei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ye",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Wenyu",
"sequence": "additional"
}
],
"container-title": "Journal of Thoracic Disease",
"container-title-short": "J Thorac Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"amegroups.com"
]
},
"created": {
"date-parts": [
[
2024,
6,
21
]
],
"date-time": "2024-06-21T06:41:12Z",
"timestamp": 1718952072000
},
"deposited": {
"date-parts": [
[
2024,
6,
29
]
],
"date-time": "2024-06-29T02:02:39Z",
"timestamp": 1719626559000
},
"indexed": {
"date-parts": [
[
2024,
6,
30
]
],
"date-time": "2024-06-30T00:16:58Z",
"timestamp": 1719706618260
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2024,
6
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2024,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
6
]
]
}
},
"link": [
{
"URL": "https://jtd.amegroups.com/article/download/87028/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8611",
"original-title": [],
"page": "3606-3622",
"prefix": "10.21037",
"published": {
"date-parts": [
[
2024,
6
]
]
},
"published-online": {
"date-parts": [
[
2024,
6
]
]
},
"published-print": {
"date-parts": [
[
2024,
6
]
]
},
"publisher": "AME Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://jtd.amegroups.com/article/view/87028/html"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and safety of casirivimab and imdevimab for preventing and treating COVID-19: a systematic review and meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.21037/ame_crossmark_policy",
"volume": "16"
}
