Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome
et al., Critical Care, doi:10.1186/s13054-020-03249-y, Aug 2020
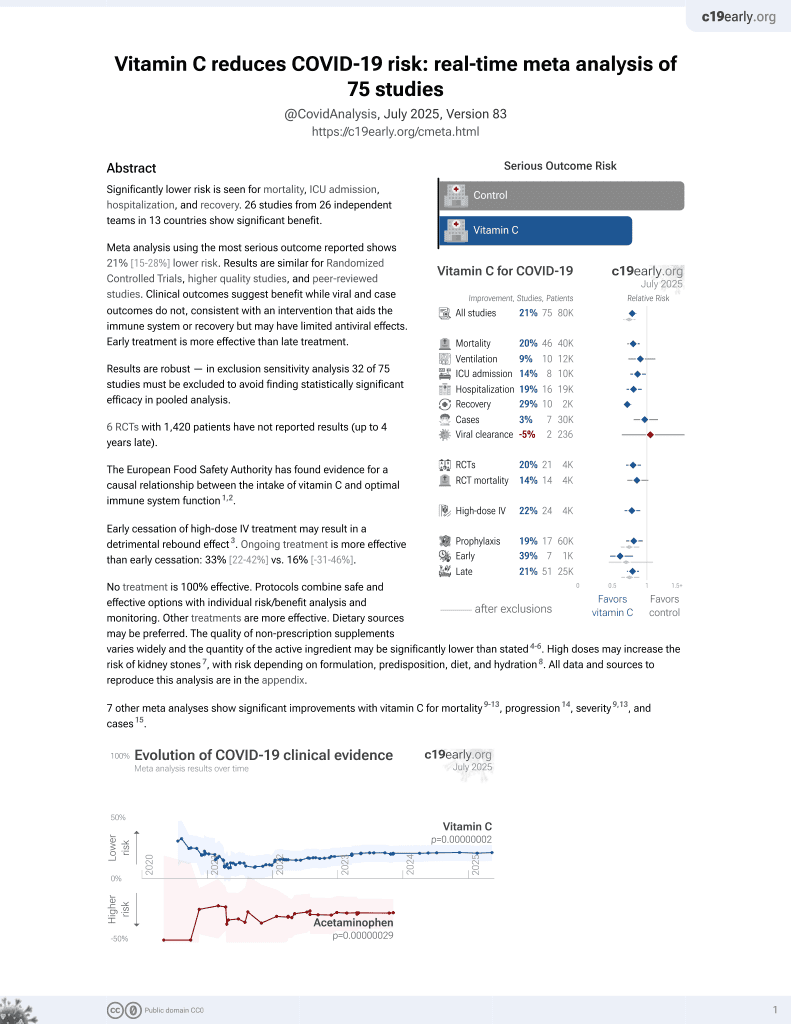
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Small study of 18 COVID-19 ARDS patients showing that vitamin C levels were very low - 17 patients had undetectable levels and one had a low level (2.4 mg/L).
Chiscano-Camón et al., 26 Aug 2020, peer-reviewed, mean age 59.0, 5 authors.
Abstract: Chiscano-Camón et al. Critical Care
(2020) 24:522
https://doi.org/10.1186/s13054-020-03249-y
RESEARCH LETTER
Open Access
Vitamin C levels in patients with SARS-CoV2-associated acute respiratory distress
syndrome
Luis Chiscano-Camón1,2,3, Juan Carlos Ruiz-Rodriguez1,2,3*, Adolf Ruiz-Sanmartin1,2, Oriol Roca1,2,3,4 and
Ricard Ferrer1,2,3,4
Vitamin C is an antioxidant with anti-inflammatory and
immune-supportive properties. Its levels are decreased
in patients with sepsis-related acute respiratory distress
syndrome (ARDS). Moreover, a significant number of
patients with severe acute respiratory syndrome
coronavirus-2 (SARS-CoV-2) disease developed ARDS
[1]. Therefore, we hypothesized that ARDS coronavirus
disease 2019 (COVID-19) patients may present vitamin
C deficiency.
Plasma vitamin C levels in a population of adult
ICU patients COVID-19 who met ARDS criteria according to the Berlin definition [2] were prospectively
measured. The study was approved by the local
Clinical Research Ethics Committee (PR (AG)270/
2020). Main characteristics of the population included
are presented in Table 1. None of the patients included presented shock or sepsis on admission.
Equally, no bacterial co-infection during their ICU
course was documented. All patients survived. Vitamin C was determined by high-performance liquid
chromatography with photodiode detector (detection
limit 1.5 mg/L). Vitamin C reference values in general
population used to be above 5 mg/L. Seventeen patients (94.4%) had undetectable vitamin C levels and
1 patient had low levels (2.4 mg/L).
To our knowledge, this is the first study to analyze the
levels of vitamin C in patients with SARS-CoV-2-associated ARDS. Our study revealed that vitamin C levels are
undetectable in more than 90% of the patients included.
The mechanisms of this significant reduction in vitamin
C are uncertain. We hypothesized that several mechanisms, such as increased metabolic consumption due to
the enhanced inflammatory response, glomerular hyperfiltration, dialysis, decreased gastrointestinal absorption,
or decreased recycling of dehydroascorbate to ascorbic
acid, may be involved.
Moreover, vitamin C may have implications for treatment of COVID-19-associated ARDS [3]. Indeed, one
preclinical study showed that vitamin C increased resistance to infection caused by coronavirus [4]. Moreover,
other clinical studies that included surgical patients and
patients with pneumonia showed encouraging results in
terms of decreased incidence and severity of lung injury
and mortality [5].
* Correspondence: jcruiz@vhebron.net
1
Intensive Care Department, Vall d’Hebron Hospital Universitari, Vall d’Hebron
Barcelona Hospital Campus, Passeig Vall d’Hebron 119-129, Barcelona 08035,
Spain
2
Shock, Organ Dysfunction and Resuscitation Research Group, Vall d’Hebron
Hospital Universitari, Vall d’Hebron Barcelona Hospital Campus, Passeig Vall
d’Hebron 119-129, Barcelona 08035, Spain
Full list of author information is available at the end of the article
© The Author(s). 2020 Open Access This article is licensed under a Creative Commons Attribution 4.0 International License,
which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if
changes were made. The images or other third party material in this article are included in the article's Creative..
DOI record:
{
"DOI": "10.1186/s13054-020-03249-y",
"ISSN": [
"1364-8535"
],
"URL": "http://dx.doi.org/10.1186/s13054-020-03249-y",
"alternative-id": [
"3249"
],
"article-number": "522",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "16 July 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 August 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "26 August 2020"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "We complied with the guidelines for human studies and our research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Information revealing the subject’s identity is to be avoided. The study was approved by the local Clinical Research Ethics Committee (PR (AG)270/2020) with exemption from informed consent."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Chiscano-Camón",
"given": "Luis",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ruiz-Rodriguez",
"given": "Juan Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruiz-Sanmartin",
"given": "Adolf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roca",
"given": "Oriol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferrer",
"given": "Ricard",
"sequence": "additional"
}
],
"container-title": "Critical Care",
"container-title-short": "Crit Care",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2020,
8,
26
]
],
"date-time": "2020-08-26T09:03:01Z",
"timestamp": 1598432581000
},
"deposited": {
"date-parts": [
[
2021,
8,
25
]
],
"date-time": "2021-08-25T23:55:17Z",
"timestamp": 1629935717000
},
"indexed": {
"date-parts": [
[
2024,
5,
7
]
],
"date-time": "2024-05-07T18:14:48Z",
"timestamp": 1715105688794
},
"is-referenced-by-count": 86,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
8,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
26
]
],
"date-time": "2020-08-26T00:00:00Z",
"timestamp": 1598400000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
8,
26
]
],
"date-time": "2020-08-26T00:00:00Z",
"timestamp": 1598400000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-020-03249-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13054-020-03249-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13054-020-03249-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2020,
8,
26
]
]
},
"published-online": {
"date-parts": [
[
2020,
8,
26
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.3633",
"doi-asserted-by": "publisher",
"key": "3249_CR1",
"unstructured": "Murthy S, Gomersall C, Fowler R. Care for critically ill patients with COVID-19. JAMA. 2020. https://doi.org/10.1001/jama.2020.3633."
},
{
"author": "VM Ranieri",
"first-page": "2526",
"issue": "23",
"journal-title": "JAMA",
"key": "3249_CR2",
"unstructured": "ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutksy AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.",
"volume": "307",
"year": "2012"
},
{
"DOI": "10.1186/s13054-020-02851-4",
"author": "A Carr",
"doi-asserted-by": "publisher",
"first-page": "133",
"journal-title": "Crit Care",
"key": "3249_CR3",
"unstructured": "Carr A. A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care. 2020;24:133.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1007/BF01317848",
"author": "JG Atherton",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "Arch Viro",
"key": "3249_CR4",
"unstructured": "Atherton JG, Kratzing CC, Fisher A. The effect of ascorbic acid on infection of chick-embryo ciliated tracheal organ cultures by coronavirus. Arch Viro. 1978;56:195–9.",
"volume": "56",
"year": "1978"
},
{
"DOI": "10.1097/00000658-200212000-00014",
"author": "AB Nathens",
"doi-asserted-by": "publisher",
"first-page": "814",
"journal-title": "Ann Surg",
"key": "3249_CR5",
"unstructured": "Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, et al. Randomized, prospective trial of antioxidant supplementation in critically III surgical patients. Ann Surg. 2002;236:814–22.",
"volume": "236",
"year": "2002"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://ccforum.biomedcentral.com/articles/10.1186/s13054-020-03249-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}