A SONAR report on Nirmatrelvir/ritonavir-associated rebound COVID-19: Using new databases for evaluating new diseases
et al., PLOS ONE, doi:10.1371/journal.pone.0308205, Sep 2024
Retrospective case series identifying 35 cases of presumed or documented paxlovid rebound COVID-19 infection from pre-print articles, social media posts, and news reports. Authors highlight a delay of 2-3 months for reports to appear in peer-reviewed literature.
Bennett et al., 25 Sep 2024, peer-reviewed, 15 authors.
Contact: charlesleebennett@gmail.com.
A SONAR report on Nirmatrelvir/ritonavir-associated rebound COVID-19: Using new databases for evaluating new diseases
PLOS ONE, doi:10.1371/journal.pone.0308205
Introduction In May 2022, the Centers for Disease Control and Prevention disseminated an alert advising that "a few" persons with Nirmatrelvir/ritonavir (NM/R)-associated rebound of COVID-19 infection had been identified. Three case reports appearing as pre-print postings described the first cases. Analyses in March 2023 by NM/R's manufacturer and the Food and Drug Administration (FDA) reported no association between NM/R and COVID-19 rebound in a large phase 3 randomized clinical trial. Our study evaluated if social media databases or electronically disseminated new articles might provide insights related to the putative new toxicity, NM/R-associated COVID-19 rebound.
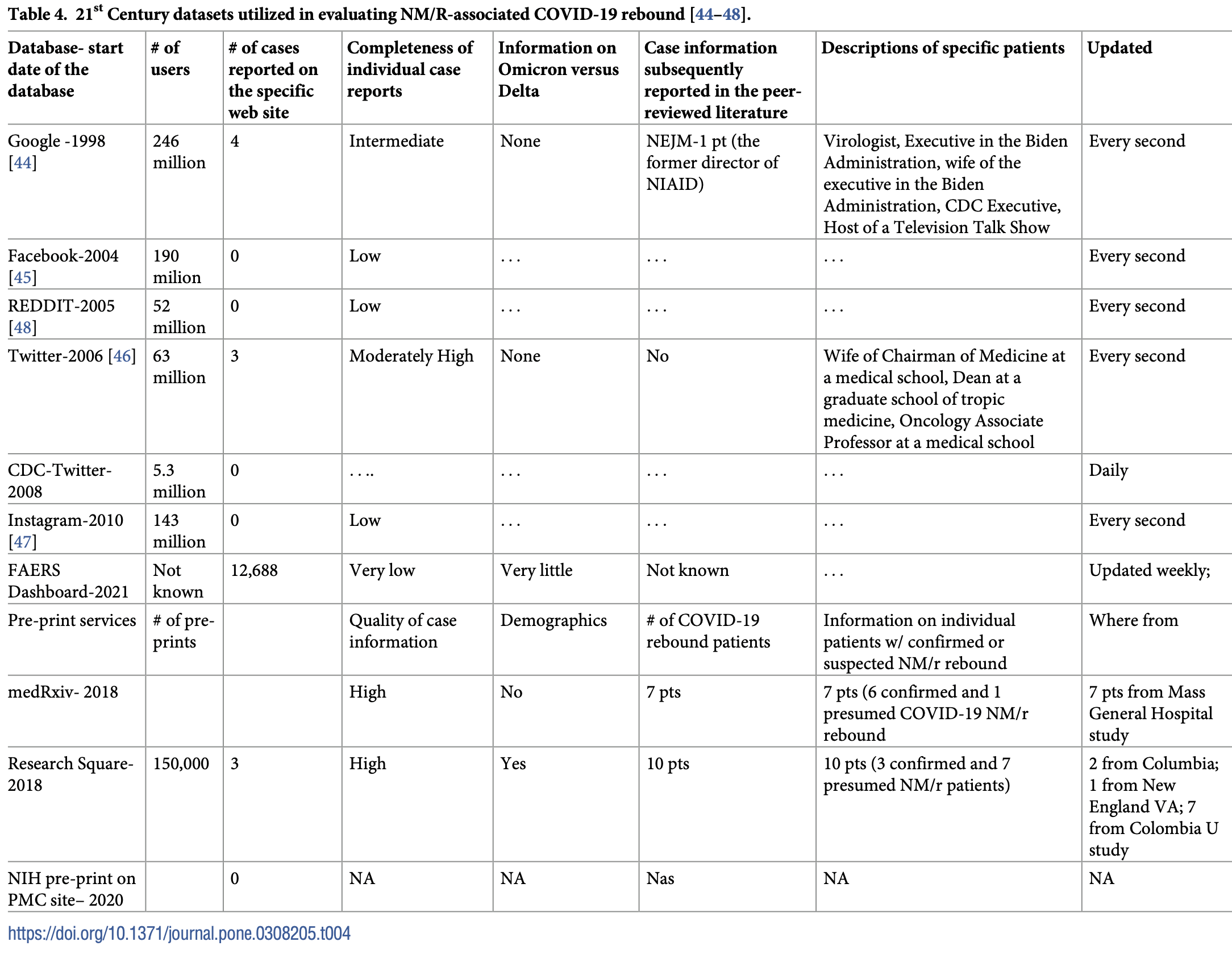
Methods Information on NM/R-associated COVID-19 rebound cases was abstracted from preprint postings of non-peer-reviewed manuscripts, social media websites, electronically disseminated print and television media reports, a new FDA adverse event database for drugs that received Emergency Use Approval, and news articles in scientific journals.
Results Thirty-five persons experienced presumed or documented NM/R-associated COVID-19 rebound, based on information described in preprint services (n = 27), Twitter postings and
Supporting information S1
References
Alshanqeeti, Bhargava, COVID-19 Rebound After Paxlovid Treatment: A Case Series and Review of Literature, Cureus, doi:10.7759/cureus.26239
Antonelli, Focosi, Turriziani, Tuccori, Brandi et al., Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.06.029
Bennett, Gundabolu, Kwak, Djulbegovic, Champigneulle et al., Using Twitter for the identification of COVID-19 vaccine-associated haematological adverse events, Lancet Haematol, doi:10.1016/S2352-3026%2821%2900378-1
Boucau, Uddin, Marino, Regan, Flynn et al., Characterization of Virologic Rebound Following Nirmatrelvir-Ritonavir Treatment for Coronavirus Disease 2019 (COVID-19), Clinical Infectious Diseases, doi:10.1093/cid/ciac512
Boucau, Uddin, Marino, Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID 19, medRxiv
Carlin, Clark, Chaillon, Garretson, Bray et al., Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment, Res Sq, doi:10.21203/rs.3.rs-1662783/v1
Charness, Gupta, Stack, Rapid relapse of symptomatic Omnicron SARS CoV2 infection following early suppression with nirmatrelvir-ritonavir, ResearchSquare
Charness, Gupta, Stack, Rapid relapse of symptomatic Omnicron SARS CoV2 infection following early suppression with nirmatrelvir-ritonavir, ResearchSquare
Charness, Gupta, Stack, Strymish, Lindy, Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment, The New England Journal of Medicine, doi:10.1056/NEJMc2206449
Choo, Ranney, Chan, Trueger, Walsh et al., Twitter as a tool for communication and knowledge exchange in academic medicine: A guide for skeptics and novices, Med Teach, doi:10.3109/0142159X.2014.993371
Chretien, Azar, Kind, Physicians on Twitter, JAMA, doi:10.1001/jama.2011.68
Coulson, Adam, Gray, Evans, COVID-19 "Rebound" associated with nirmatrelvir-ritonavir, medRxiv. Research Square
Coulson, Adams, Gray, Evans, COVID-19 "Rebound" associated with nirmatrelvir/ritonavir pre-hospital therapy, J Infect, doi:10.1016/j.jinf.2022.06.011
Dai, Lee, Nathanson, Leonelli, Petros et al., Viral Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Infection in mRNA-Vaccinated Individuals Treated and Not Treated with Nirmatrelvir-Ritonavir, medRxiv, doi:10.1101/2022.08.04.22278378
Daily, Briefing, Fauci says he believes Paxlovid kept him out of the hospital, even though he tested positive again, The New York Times
Desk, First lady Jill Biden tests positive for COVID-19 in rebound case, NPR
Epling, Rocco, Boswell, COVID-19 redux: clinicial, virologic, and immunologic evaluation after nirmatrelvir-ritonavir, medRxiv
Epling, Rocco, Boswell, Laidlaw, Galindo et al., Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment, Clinical Infectious Diseases, doi:10.1093/cid/ciac663
Foley, Ld, Biden experiences a COVID rebound after treatment with one course of Paxlovid, Politico
Fraser, Brierley, Dey, Polka, Palfy et al., The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape, PLoS Biol, doi:10.1371/journal.pbio.3000959
Goodman, Fauci says his Covid rebounded after Paxlovid, CNN
Hotez, Twitter Thread
Jay, Jennifer, Emily, Mira, The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants, medRxiv
Judd, Cole, Biden still testing positive after rebound COVID-19 case but 'continues to feel well, CNN
Kaplan, Coronavirus today: Is the Paxlovid rebound real, The LA Times
Li, Zhang, Liu, Wang, Liu, Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS, Pharmaceuticals, doi:10.3390/ph15121455
Lu, Kessler, Schulz, Bian, Chen et al., Systematic approach to pharmacovigilance beyond the limits: the southern network on adverse reactions (SONAR) projects, Adv Pharmacoepidemiol Drug Saf
Mcphillips, COVID-19 rebound is probably more common than data suggests, but Paxlovid is still effective, CNN
Muir, Kallam, Koepsell, Gundabolu, Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination, The New England journal of medicine, doi:10.1056/NEJMc2105869
Pandit, Radin, Chiang, Spencer, Pawelek et al., The Coronavirus Disease 2019 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated With Nirmatrelvir Plus Ritonavir Versus Untreated Controls, Clin Infect Dis, doi:10.1093/cid/ciad102
Pershad, Hangge, Albadawi, Oklu, Social Medicine: Twitter in Healthcare, J Clin Med, doi:10.3390/jcm7060121
Rubin, From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid, JAMA, doi:10.1001/jama.2022.9925
Saad, Stephen Colbert gets COVID-19 again, cancels 'Late Show, The LA Times
Scribner, Biden negative for COVID after weeklong "rebound" case
Sheehan, CDC boss tests positive for Covid AGAIN: Rochelle Walensky is diagnosed with virus just days after being given the all-clear, The Daily Mail
Smith-Schoenwalder, Biden tests positive for 7th straight day after 'Rebound' COVID-19 infection, US News
Soares, Bm, Cardin, Leister-Tebbe, Zhu et al., Viral Load Rebound in Placebo and Nirmatrelvir-Ritonavir Treated COVID-19 Patients is not Associated with Recurrence of Severe Disease or Mutations
Thompson, Majhail, Wood, Perales, Chaboissier, Social Media and the Practicing Hematologist: Twitter 101 for the Busy Healthcare Provider, Curr Hematol Malig Rep, doi:10.1007/s11899-015-0286-x
Wang, Berger, Davis, Kaelber, Volkow et al., COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022, medRxiv, doi:10.1101/2022.06.21.22276724
White House ; Kevin, Connor, An update from Dr
DOI record:
{
"DOI": "10.1371/journal.pone.0308205",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0308205",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Introduction</jats:title>\n<jats:p>In May 2022, the Centers for Disease Control and Prevention disseminated an alert advising that “a few” persons with Nirmatrelvir/ritonavir (NM/R)-associated rebound of COVID-19 infection had been identified. Three case reports appearing as pre-print postings described the first cases. Analyses in March 2023 by NM/R’s manufacturer and the Food and Drug Administration (FDA) reported no association between NM/R and COVID-19 rebound in a large phase 3 randomized clinical trial. Our study evaluated if social media databases or electronically disseminated new articles might provide insights related to the putative new toxicity, NM/R-associated COVID-19 rebound.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods</jats:title>\n<jats:p>Information on NM/R-associated COVID-19 rebound cases was abstracted from preprint postings of non-peer-reviewed manuscripts, social media websites, electronically disseminated print and television media reports, a new FDA adverse event database for drugs that received Emergency Use Approval, and news articles in scientific journals.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Results</jats:title>\n<jats:p>Thirty-five persons experienced presumed or documented NM/R-associated COVID-19 rebound, based on information described in preprint services (n = 27), Twitter postings and related news articles (n = 7), and news articles without related Twitter reports (n = 1). These reports included information on dates of initial COVID-19 illness and rebound onset, COVID-19 testing, vaccine status, presentation, and outcome. A new FDA safety database identified 12,500 possible cases of this toxicity, but the quality of these data was poor. Preprint postings preceded peer-reviewed publications describing the same cases by four months. Social media websites including Instagram, Reddit, YouTube, the Center for Disease Control and Prevention’s (CDC) Health Alert Network, CDC Twitter, and Facebook did not provide clinically meaningful information on individual cases.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Conclusion</jats:title>\n<jats:p>Preprint services and Twitter facilitated identification of the largest case series of NM/R-associated COVID-19 rebound. The cases were reported in non-peer-reviewed media several weeks prior to the first peer-reviewed electronically disseminated publication of one person with this diagnosis.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8645-5705",
"affiliation": [],
"authenticated-orcid": true,
"family": "Bennett",
"given": "Charles L.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Magagnoli",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gundabolu",
"given": "Krishna",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4113-6655",
"affiliation": [],
"authenticated-orcid": true,
"family": "Georgantopoulos",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebby",
"given": "Akida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Watson",
"given": "Gretchen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Knopf",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carson",
"given": "Kenneth R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hrushesky",
"given": "William J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nabhan",
"given": "Chadi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zyszkowski",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Edward B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gale",
"given": "Robert Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosen",
"given": "Steven T.",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2024,
9,
25
]
],
"date-time": "2024-09-25T19:27:40Z",
"timestamp": 1727292460000
},
"deposited": {
"date-parts": [
[
2024,
9,
25
]
],
"date-time": "2024-09-25T19:28:01Z",
"timestamp": 1727292481000
},
"editor": [
{
"affiliation": [],
"family": "Rojekar",
"given": "Satish",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100000054",
"award": [
"1R01 CA102713"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100000054",
"id-type": "DOI"
}
],
"name": "National Cancer Institute"
},
{
"name": "Beckman Research Institute and the City of Hope Comprehensive Cancer Center, Duarte, California"
},
{
"name": "Beckman Research Institute and the City of Hope Comprehensive Cancer Center, Duarte, California"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
25
]
],
"date-time": "2024-09-25T20:10:10Z",
"timestamp": 1727295010597
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2024,
9,
25
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2024,
9,
25
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
25
]
],
"date-time": "2024-09-25T00:00:00Z",
"timestamp": 1727222400000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0308205",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0308205",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2024,
9,
25
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
25
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"article-title": "Systematic approach to pharmacovigilance beyond the limits: the southern network on adverse reactions (SONAR) projects",
"author": "ZK Lu",
"issue": "2",
"journal-title": "Adv Pharmacoepidemiol Drug Saf",
"key": "pone.0308205.ref001",
"volume": "3",
"year": "2014"
},
{
"DOI": "10.1056/NEJMc2105869",
"article-title": "Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination",
"author": "KL Muir",
"doi-asserted-by": "crossref",
"first-page": "1964",
"issue": "20",
"journal-title": "The New England journal of medicine",
"key": "pone.0308205.ref002",
"volume": "384",
"year": "2021"
},
{
"article-title": "Viral Kinetics of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Omicron Infection in mRNA-Vaccinated Individuals Treated and Not Treated with Nirmatrelvir-Ritonavir",
"author": "EY Dai",
"journal-title": "medRxiv",
"key": "pone.0308205.ref003",
"year": "2022"
},
{
"article-title": "The Paxlovid Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences Between Paxlovid and Untreated COVID-19 Participants",
"author": "AP Jay",
"journal-title": "medRxiv",
"key": "pone.0308205.ref004",
"year": "2022"
},
{
"article-title": "COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022",
"author": "L Wang",
"journal-title": "medRxiv",
"key": "pone.0308205.ref005",
"year": "2022"
},
{
"key": "pone.0308205.ref006",
"unstructured": "CDC Health Advisory. COVID-19 rebound after Paxlovid treatment. In: CDC Health Alert Network. emergency.cdc.gov: CDC; 2022 p. 4. https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf (accessed 9/1/2024)."
},
{
"article-title": "Virologic and Immunologic Characterization of COVID-19 Recrudescence after Nirmatrelvir/Ritonavir Treatment",
"author": "AF Carlin",
"journal-title": "Res Sq",
"key": "pone.0308205.ref007",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2206449",
"article-title": "Rebound of SARS-CoV-2 Infection after Nirmatrelvir-Ritonavir Treatment",
"author": "ME Charness",
"doi-asserted-by": "crossref",
"first-page": "1045",
"issue": "11",
"journal-title": "The New England Journal of Medicine",
"key": "pone.0308205.ref008",
"volume": "387",
"year": "2022"
},
{
"key": "pone.0308205.ref009",
"unstructured": "FDA. FDA launches COVID-19 EUA FAERS public dashboard. Twitter.com; 2021."
},
{
"DOI": "10.1093/cid/ciad102",
"article-title": "The Coronavirus Disease 2019 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated With Nirmatrelvir Plus Ritonavir Versus Untreated Controls",
"author": "JA Pandit",
"doi-asserted-by": "crossref",
"first-page": "25",
"issue": "1",
"journal-title": "Clin Infect Dis",
"key": "pone.0308205.ref010",
"volume": "77",
"year": "2023"
},
{
"key": "pone.0308205.ref011",
"unstructured": "FDA Adverse Event Reporting System (FAERS) Public Dashboard [Internet]. FDA. 2021 [cited 04/09/23]. https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard."
},
{
"article-title": "COVID-19 Rebound After Paxlovid Treatment: A Case Series and Review of Literature",
"author": "S Alshanqeeti",
"first-page": "e26239",
"issue": "6",
"journal-title": "Cureus",
"key": "pone.0308205.ref012",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.cmi.2022.06.029",
"article-title": "Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation",
"author": "G Antonelli",
"doi-asserted-by": "crossref",
"first-page": "1657",
"issue": "12",
"journal-title": "Clin Microbiol Infect",
"key": "pone.0308205.ref013",
"volume": "28",
"year": "2022"
},
{
"key": "pone.0308205.ref014",
"unstructured": "Axios. Executive in the Biden Administration tests positive for “rebound” COVID. www.Twitter.com: Twitter; 2022."
},
{
"DOI": "10.1093/cid/ciac512",
"article-title": "Characterization of Virologic Rebound Following Nirmatrelvir-Ritonavir Treatment for Coronavirus Disease 2019 (COVID-19)",
"author": "J Boucau",
"doi-asserted-by": "crossref",
"first-page": "e526",
"issue": "3",
"journal-title": "Clinical Infectious Diseases",
"key": "pone.0308205.ref015",
"volume": "76",
"year": "2023"
},
{
"key": "pone.0308205.ref016",
"unstructured": "Brenda Goodman VL. Fauci says his Covid rebounded after Paxlovid CNN 2022 06/30/22 (col. 2022)."
},
{
"key": "pone.0308205.ref017",
"unstructured": "Smith-Schoenwalder C. Biden tests positive for 7th straight day after ‘Rebound’ COVID-19 infection. US News. 2022 08/05/22."
},
{
"key": "pone.0308205.ref018",
"unstructured": "FDA. FDA briefing document for nirmatrelvir (NDA# 217188). March 16, 2023. https://www.fda.gov/media/166197/download (accessed 9/1/2024)"
},
{
"DOI": "10.1016/j.jinf.2022.06.011",
"article-title": "COVID-19 \"Rebound\" associated with nirmatrelvir/ritonavir pre-hospital therapy",
"author": "JM Coulson",
"doi-asserted-by": "crossref",
"first-page": "436",
"issue": "4",
"journal-title": "J Infect",
"key": "pone.0308205.ref019",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac663",
"article-title": "Clinical, Virologic, and Immunologic Evaluation of Symptomatic Coronavirus Disease 2019 Rebound Following Nirmatrelvir/Ritonavir Treatment",
"author": "BP Epling",
"doi-asserted-by": "crossref",
"first-page": "573",
"issue": "4",
"journal-title": "Clinical Infectious Diseases",
"key": "pone.0308205.ref020",
"volume": "76",
"year": "2023"
},
{
"key": "pone.0308205.ref021",
"unstructured": "Foley KE. LD. Biden experiences a COVID rebound after treatment with one course of Paxlovid. Politico. 2022 07/30/22."
},
{
"key": "pone.0308205.ref022",
"unstructured": "The White House. An update from Dr. Kevin O’Connor, Physician to the President. www.Twitter.com: Twitter; 2022."
},
{
"key": "pone.0308205.ref023",
"unstructured": "The Associated Press. First lady Jill Biden has “rebound” COVID-19 case, President Joe Biden still testing negative PBS News. 2022 08/24/22."
},
{
"DOI": "10.1001/jama.2022.9925",
"article-title": "From Positive to Negative to Positive Again-The Mystery of Why COVID-19 Rebounds in Some Patients Who Take Paxlovid",
"author": "R. Rubin",
"doi-asserted-by": "crossref",
"first-page": "2380",
"issue": "24",
"journal-title": "JAMA",
"key": "pone.0308205.ref024",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.21203/rs.3.rs-1720472/v2",
"doi-asserted-by": "crossref",
"key": "pone.0308205.ref025",
"unstructured": "Soares H. BM, Cardin R, Leister-Tebbe H., Zhu Y., Guan S., et al. Viral Load Rebound in Placebo and Nirmatrelvir-Ritonavir Treated COVID-19 Patients is not Associated with Recurrence of Severe Disease or Mutations. 2022."
},
{
"key": "pone.0308205.ref026",
"unstructured": "Hotez P. Twitter Thread. www.Twitter.com: Twitter; 2022."
},
{
"key": "pone.0308205.ref027",
"unstructured": "Judd D, Cole D. Biden still testing positive after rebound COVID-19 case but ‘continues to feel well,’ White House says. CNN. August 1, 2022. https://www.cnn.com/2022/07/31/politics/joe-biden-covid-positive-day-two/index.html (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref028",
"unstructured": "Scribner H. Biden negative for COVID after weeklong “rebound” case. Axios. 8/6/2022. https://www.axios.com/2022/08/06/joe-biden-covid-19-case-negative (accessed 9/1/2024)"
},
{
"key": "pone.0308205.ref029",
"unstructured": "McPhillips E. COVID-19 rebound is probably more common than data suggests, but Paxlovid is still effective. CNN. 2022 08/26/22. https://www.cnn.com/2022/08/26/health/paxlovid-rebound-how-common/index.html (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref030",
"unstructured": "Washington Desk. First lady Jill Biden tests positive for COVID-19 in rebound case. NPR. 2022 08/24/22. https://www.npr.org/2022/08/24/1119262837/first-lady-jill-biden-tests-positive-covid-19-paxlovid-rebound (accessed 9/1/2024)"
},
{
"key": "pone.0308205.ref031",
"unstructured": "The Associated Press Staff. US First Lady Jill Biden tests negative for COVID after rebound. CTV News. 08/29/22. https://www.ctvnews.ca/mobile/health/coronavirus/u-s-first-lady-jill-biden-tests-negative-for-covid-after-rebound-1.6047745?cache=yesclipId10406200text/html;charset=utf-80404/7.314145/7.314145 (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref032",
"unstructured": "Daily Covid Briefing. Fauci says he believes Paxlovid kept him out of the hospital, even though he tested positive again. The New York Times 2022 06/29/22. https://www.nytimes.com/live/2022/06/29/world/covid-19-mandates-vaccine-cases (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref033",
"unstructured": "Kaplan K. Coronavirus today: Is the Paxlovid rebound real. The LA Times. 2022 11/15/22. https://www.latimes.com/science/newsletter/2022-11-15/coronavirus-today-paxlovid-rebound-coronavirus-today (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref034",
"unstructured": "Sheehan M. CDC boss tests positive for Covid AGAIN: Rochelle Walensky is diagnosed with virus just days after being given the all-clear. The Daily Mail. 2022 10/31/22. https://www.dailymail.co.uk/health/article-11374893/CDC-boss-tests-positive-Covid-Rochelle-Walensky-suffers-Paxlovid-rebound.html (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref035",
"unstructured": "Saad N. Stephen Colbert gets COVID-19 again, cancels ‘Late Show.’ The LA Times. 2022 05/09/22. https://www.latimes.com/entertainment-arts/tv/story/2022-05-09/stephen-colbert-covid-19-recurrence-cancels-late-show-episodes (accessed 9/1/2024)."
},
{
"key": "pone.0308205.ref036",
"unstructured": "The Associated Press. CDC Director Rochelle Walensky tests positive for COVID again after taking a course of the anti-viral Paxlovid. NBC News. 2022 10/31/22. https://www.nbcnews.com/news/us-news/cdc-director-tests-positive-covid-paxlovid-rebound-case-rcna54870 accessed 9/1/2024)."
},
{
"article-title": "Adverse Events Associated with Nirmatrelvir/Ritonavir: A Pharmacovigilance Analysis Based on FAERS",
"author": "M Li",
"issue": "12",
"journal-title": "Pharmaceuticals (Basel)",
"key": "pone.0308205.ref037",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.1371/journal.pbio.3000959",
"article-title": "The evolving role of preprints in the dissemination of COVID-19 research and their impact on the science communication landscape",
"author": "N Fraser",
"doi-asserted-by": "crossref",
"first-page": "e3000959",
"issue": "4",
"journal-title": "PLoS Biol",
"key": "pone.0308205.ref038",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1001/jama.2011.68",
"article-title": "Physicians on Twitter",
"author": "KC Chretien",
"doi-asserted-by": "crossref",
"first-page": "566",
"issue": "6",
"journal-title": "JAMA",
"key": "pone.0308205.ref039",
"volume": "305",
"year": "2011"
},
{
"DOI": "10.3109/0142159X.2014.993371",
"article-title": "Twitter as a tool for communication and knowledge exchange in academic medicine: A guide for skeptics and novices",
"author": "EK Choo",
"doi-asserted-by": "crossref",
"first-page": "411",
"issue": "5",
"journal-title": "Med Teach",
"key": "pone.0308205.ref040",
"volume": "37",
"year": "2015"
},
{
"DOI": "10.3390/jcm7060121",
"article-title": "Social Medicine: Twitter in Healthcare",
"author": "Y Pershad",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "J Clin Med",
"key": "pone.0308205.ref041",
"volume": "7",
"year": "2018"
},
{
"DOI": "10.1007/s11899-015-0286-x",
"article-title": "Social Media and the Practicing Hematologist: Twitter 101 for the Busy Healthcare Provider",
"author": "MA Thompson",
"doi-asserted-by": "crossref",
"first-page": "405",
"issue": "4",
"journal-title": "Curr Hematol Malig Rep",
"key": "pone.0308205.ref042",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.1016/S2352-3026(21)00378-1",
"article-title": "Using Twitter for the identification of COVID-19 vaccine-associated haematological adverse events",
"author": "CL Bennett",
"doi-asserted-by": "crossref",
"first-page": "e12",
"issue": "1",
"journal-title": "Lancet Haematol",
"key": "pone.0308205.ref043",
"volume": "9",
"year": "2022"
},
{
"key": "pone.0308205.ref044",
"unstructured": "Worldwide visits to Google.com from November 2022 to April 2023 (in billions) [Internet]. SimilarWeb. 2023 [cited October 13, 2023]. https://www.statista.com/statistics/268252/web-visitor-traffic-to-googlecom/."
},
{
"key": "pone.0308205.ref045",
"unstructured": "Facebook Users, Stats, Data & Trends [Internet]. Datareportal.com. 2023 [cited October 13, 2023]. https://datareportal.com/essential-facebook-stats."
},
{
"key": "pone.0308205.ref046",
"unstructured": "Twitter Users, Stats, Data & Trends [Internet]. Datareportal.com. 2023 [cited October 13, 2023]. https://datareportal.com/essential-twitter-stats."
},
{
"key": "pone.0308205.ref047",
"unstructured": "Instagram Users, Stats, Data & Trends [Internet]. Datareportal.com. 2023 [cited October 13, 2023]. https://datareportal.com/essential-instagram-stats."
},
{
"key": "pone.0308205.ref048",
"unstructured": "Traffic Analytics: reddit.com [Internet]. Semrush.com. 2023 [cited Oct 13, 2023]. https://www.semrush.com/analytics/traffic/overview/?searchType=domain&q=reddit.com."
},
{
"DOI": "10.21203/rs.3.rs-1588371/v3",
"doi-asserted-by": "crossref",
"key": "pone.0308205.ref049",
"unstructured": "Charness M, Gupta, Stack J et al. Rapid relapse of symptomatic Omnicron SARS CoV2 infection following early suppression with nirmatrelvir-ritonavir. (Version 1). ResearchSquare. April 26, 2022."
},
{
"DOI": "10.21203/rs.3.rs-1588371/v3",
"doi-asserted-by": "crossref",
"key": "pone.0308205.ref050",
"unstructured": "Charness M, Gupta K, Stack J et al. Rapid relapse of symptomatic Omnicron SARS CoV2 infection following early suppression with nirmatrelvir-ritonavir. (Version 2). ResearchSquare May 13, 2022."
},
{
"article-title": "COVID-19 redux: clinicial, virologic, and immunologic evaluation after nirmatrelvir-ritonavir",
"author": "BP Epling",
"journal-title": "medRxiv",
"key": "pone.0308205.ref051",
"year": "2022"
},
{
"article-title": "Virologic characterization of symptom rebound following nirmatrelvir-ritonavir treatment for COVID 19",
"author": "J Boucau",
"journal-title": "medRxiv",
"key": "pone.0308205.ref052",
"year": "2022"
},
{
"article-title": "COVID-19 “Rebound” associated with nirmatrelvir-ritonavir",
"author": "JM Coulson",
"journal-title": "medRxiv",
"key": "pone.0308205.ref053",
"year": "2022"
}
],
"reference-count": 53,
"references-count": 53,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0308205"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A SONAR report on Nirmatrelvir/ritonavir-associated rebound COVID-19: Using new databases for evaluating new diseases",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "19"
}