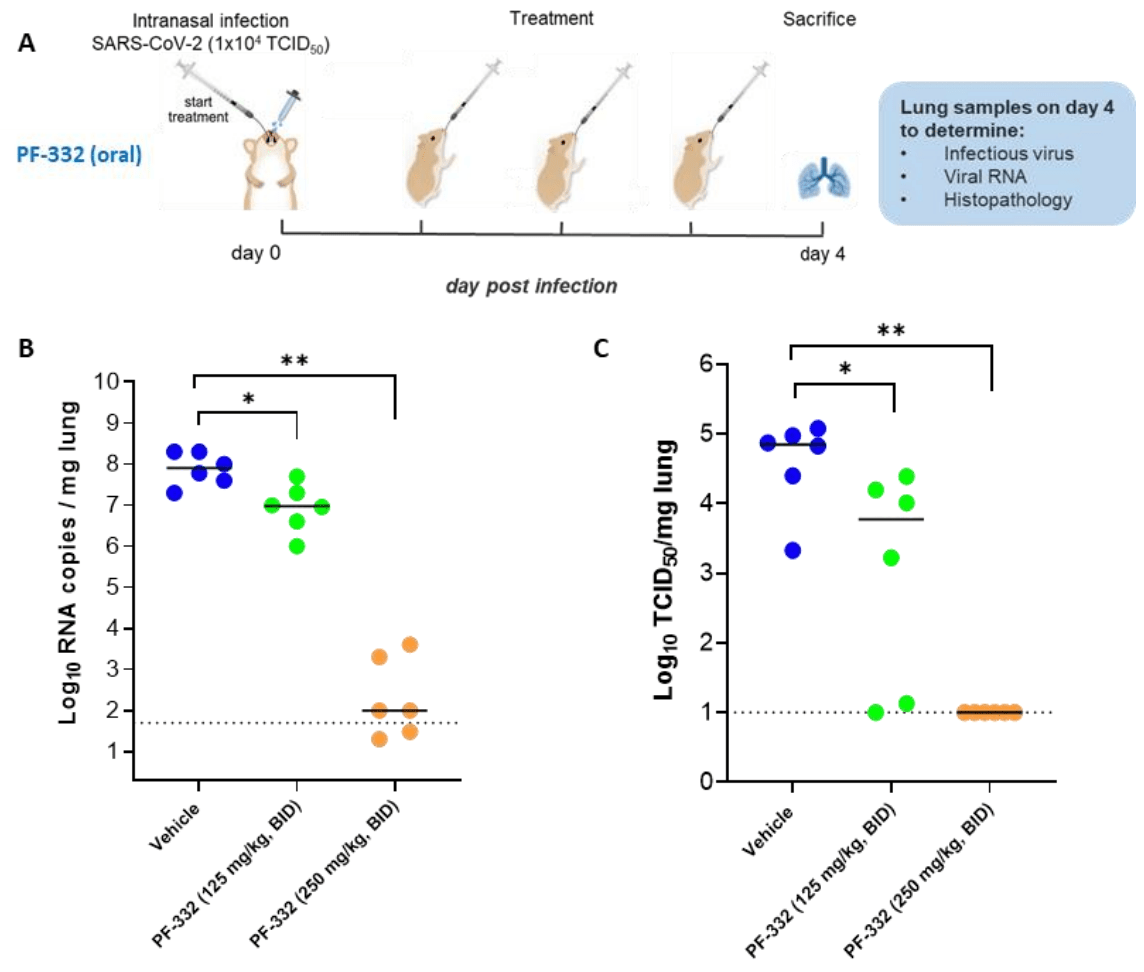
The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern
et al., bioRxiv, doi:10.1101/2021.11.04.467077, Nov 2021
In vitro and hamster study showing paxlovid component PF-07321332 effective against four variants of concern, inibits replication of the alpha variant in primary human airway epithelial cells, protected Syrian hamsters against intranasal infection with B.1.351 and B.1.617.2, and prevented transmission from B.1.617.2 infected animals.
Abdelnabi et al., 5 Nov 2021, preprint, 17 authors.
The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern
doi:10.1101/2021.11.04.467077
There is an urgent need for potent and selective antivirals against SARS-CoV-2. Pfizer developed PF-07321332 (PF-332), a potent inhibitor of the viral main protease (Mpro, 3CLpro) that can be dosed orally and that is in clinical development. We here report that PF-332 exerts equipotent in vitro activity against the four SARS-CoV-2 variants of concerns (VoC) and that it can completely arrest replication of the alpha variant in primary human airway epithelial cells grown at the air-liquid interface. Treatment of Syrian Golden hamsters with PF-332 (250 mg/kg, twice daily) completely protected the animals against intranasal infection with the beta (B.1.351) and delta (B.1.617.2) SARS-CoV-2 variants. Moreover, treatment of SARS-CoV-2 (B.1.617.2) infected animals with PF-332 completely prevented transmission to untreated co-housed sentinels.
Ethics Housing conditions and experimental procedures were done with the approval and under the guidelines of the ethics committee of animal experimentation of KU Leuven (license P065-2020).
Conflict of interest
None to declare
Author
References
Abdelnabi, Comparing infectivity and virulence of emerging SARS-CoV-2 variants in Syrian hamsters, EBioMedicine
Abdelnabi, Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model, J. Infect. Dis
Abdelnabi, The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model, EBioMedicine
Boras, Discovery of a Novel Inhibitor of Coronavirus 3CL Protease for the Potential Treatment of COVID-19, bioRxiv, doi:10.1101/2020.09.12.293498
Boudewijns, STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters, Nat. Commun
Chen, Liu, Guo, Emerging coronaviruses: Genome structure, replication, and pathogenesis, Journal of Medical Virology
Do, A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents, Antiviral Res
Fan, The substrate specificity of SARS coronavirus 3C-like proteinase, Biochem. Biophys. Res. Commun
Ivens, Development of a homogeneous screening assay for automated detection of antiviral agents active against severe acute respiratory syndrome-associated coronavirus, J. Virol. Methods
Jin, Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors, Nature
Jochmans, Leyssen, Neyts, A novel method for high-throughput screening to quantify antiviral activity against viruses that induce limited CPE, J. Virol. Methods
Kabinger, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat. Struct. Mol. Biol
Kaptein, Favipiravir at high doses has potent antiviral activity in SARS-CoV-2−infected hamsters, whereas hydroxychloroquine lacks activity, Proc. Natl. Acad. Sci. U. S. A
Keizer, Van Benten, Beijnen, Schellens, Huitema, Piraña and PCluster: A modeling environment and cluster infrastructure for NONMEM, Comput. Methods Programs Biomed
Kokic, Mechanism of SARS-CoV-2 polymerase stalling by remdesivir, Nat. Commun
Lindbom, Ribbing, Jonsson, Perl-speaks-NONMEM (PsN) -A Perl module for NONMEM related programming, Comput. Methods Programs Biomed
Owen, An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19, Science, doi:10.1126/science.abl4784
Pillaiyar, Manickam, Namasivayam, Hayashi, Jung, An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: Peptidomimetics and small molecule chemotherapy, Journal of Medicinal Chemistry
Schäfer, Therapeutic efficacy of an oral nucleoside analog of remdesivir against SARS-CoV-2 pathogenesis in mice, bioRxiv : the preprint server for biology, doi:10.1101/2021.09.13.460111
Ullrich, Nitsche, The SARS-CoV-2 main protease as drug target, Bioorganic and Medicinal Chemistry Letters
DOI record:
{
"DOI": "10.1101/2021.11.04.467077",
"URL": "http://dx.doi.org/10.1101/2021.11.04.467077",
"abstract": "<jats:title>Abstract</jats:title><jats:p>There is an urgent need for potent and selective antivirals against SARS-CoV-2. Pfizer developed PF-07321332 (PF-332), a potent inhibitor of the viral main protease (Mpro, 3CLpro) that can be dosed orally and that is in clinical development. We here report that PF-332 exerts equipotent <jats:italic>in vitro</jats:italic> activity against the four SARS-CoV-2 variants of concerns (VoC) and that it can completely arrest replication of the alpha variant in primary human airway epithelial cells grown at the air-liquid interface. Treatment of Syrian Golden hamsters with PF-332 (250 mg/kg, twice daily) completely protected the animals against intranasal infection with the beta (B.1.351) and delta (B.1.617.2) SARS-CoV-2 variants. Moreover, treatment of SARS-CoV-2 (B.1.617.2) infected animals with PF-332 completely prevented transmission to untreated co-housed sentinels.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
11,
5
]
]
},
"author": [
{
"affiliation": [],
"family": "Abdelnabi",
"given": "Rana",
"sequence": "first"
},
{
"affiliation": [],
"family": "Foo",
"given": "Caroline S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9265-6028",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jochmans",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vangeel",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Jonghe",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Augustijns",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mols",
"given": "Raf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weynand",
"given": "Birgit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wattanakul",
"given": "Thanaporn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoglund",
"given": "Richard M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarning",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mowbray",
"given": "Charles E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sjö",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Escudié",
"given": "Fanny",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scandale",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chatelain",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neyts",
"given": "Johan",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
5
]
],
"date-time": "2021-11-05T22:15:12Z",
"timestamp": 1636150512000
},
"deposited": {
"date-parts": [
[
2022,
5,
25
]
],
"date-time": "2022-05-25T19:50:24Z",
"timestamp": 1653508224000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2024,
3,
2
]
],
"date-time": "2024-03-02T00:24:36Z",
"timestamp": 1709339076428
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2021,
11,
5
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.11.04.467077",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
11,
5
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
11,
5
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1002/jmv.25681",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.1"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "2021110905200473000_2021.11.04.467077v1.2",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.5b01461",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.3"
},
{
"DOI": "10.1016/j.bbrc.2005.02.061",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.4"
},
{
"DOI": "10.1016/j.bmcl.2020.127377",
"doi-asserted-by": "crossref",
"key": "2021110905200473000_2021.11.04.467077v1.5",
"unstructured": "Ullrich, S. & Nitsche, C. The SARS-CoV-2 main protease as drug target. Bioorganic and Medicinal Chemistry Letters 30, (2020)."
},
{
"DOI": "10.1101/2020.09.12.293498",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.6"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.7"
},
{
"DOI": "10.1016/j.antiviral.2021.105122",
"doi-asserted-by": "crossref",
"key": "2021110905200473000_2021.11.04.467077v1.8",
"unstructured": "Do, T. N. D. et al. A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents. Antiviral Res. 192, (2021)."
},
{
"author": "Food and Drug Administration (FDA). Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment",
"first-page": "6",
"journal-title": "Press Announcements",
"key": "2021110905200473000_2021.11.04.467077v1.9",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-20542-0",
"doi-asserted-by": "crossref",
"key": "2021110905200473000_2021.11.04.467077v1.10",
"unstructured": "Kokic, G. et al. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 12, (2021)."
},
{
"DOI": "10.1101/2021.09.13.460111",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.11"
},
{
"DOI": "10.1038/s41594-021-00651-0",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.12"
},
{
"DOI": "10.1016/j.ebiom.2021.103595",
"doi-asserted-by": "crossref",
"key": "2021110905200473000_2021.11.04.467077v1.13",
"unstructured": "Abdelnabi, R. et al. The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model. EBioMedicine 72, (2021)."
},
{
"DOI": "10.1093/infdis/jiab361",
"article-title": "Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model",
"doi-asserted-by": "crossref",
"first-page": "749",
"journal-title": "J. Infect. Dis",
"key": "2021110905200473000_2021.11.04.467077v1.14",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2014441117",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.15"
},
{
"DOI": "10.1016/j.ebiom.2021.103403",
"article-title": "Comparing infectivity and virulence of emerging SARS-CoV-2 variants in Syrian hamsters",
"doi-asserted-by": "crossref",
"first-page": "103403",
"journal-title": "EBioMedicine",
"key": "2021110905200473000_2021.11.04.467077v1.16",
"volume": "68",
"year": "2021"
},
{
"DOI": "10.1016/j.jviromet.2005.05.010",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.17"
},
{
"DOI": "10.1016/j.jviromet.2012.04.011",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.18"
},
{
"DOI": "10.1038/s41467-020-19684-y",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.19"
},
{
"DOI": "10.1016/j.cmpb.2003.11.003",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.20"
},
{
"DOI": "10.1016/j.cmpb.2010.04.018",
"doi-asserted-by": "publisher",
"key": "2021110905200473000_2021.11.04.467077v1.21"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1038/s41467-022-28354-0",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://biorxiv.org/lookup/doi/10.1101/2021.11.04.467077"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern",
"type": "posted-content"
}