Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection
et al., JAMA, doi:10.1001/jama.2021.11517, ACTION, NCT04332107, Aug 2021
RCT 263 COVID-19 outpatients showing no significant difference in COVID-19 symptoms at day 14 with a single 1.2g dose of azithromycin vs placebo. Treatment was very late, a median of 7 days after symptom onset (3 days from onset to test results, 3 days to enrollment, 1 day for shipping).
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
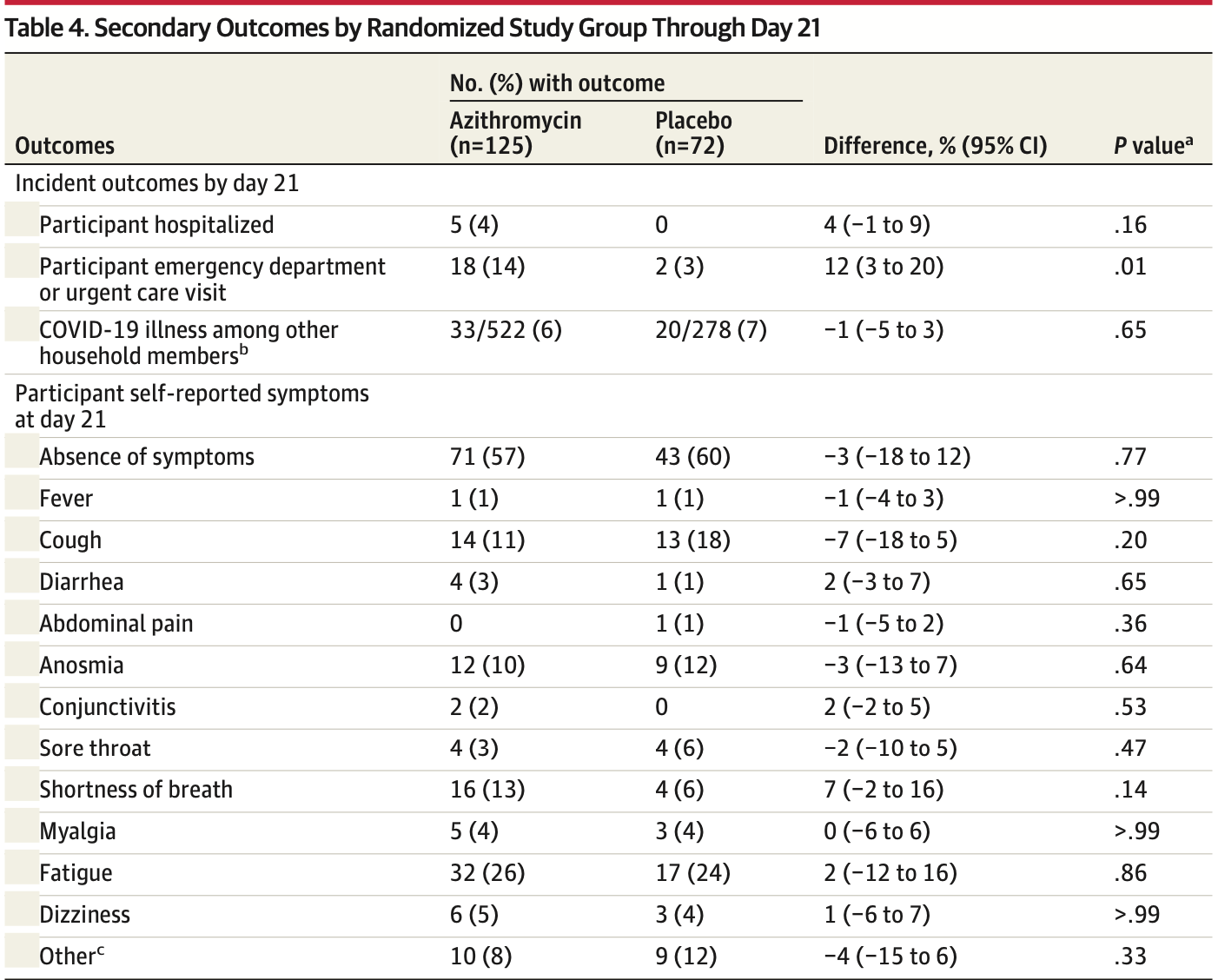
risk of hospitalization, 788.0% higher, RR 8.88, p = 0.16, treatment 5 of 125 (4.0%), control 0 of 72 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 21.
|
|
ED visit, 418.4% higher, RR 5.18, p = 0.01, treatment 18 of 125 (14.4%), control 2 of 72 (2.8%), ED visit or urgent care, day 21.
|
|
risk of no recovery, 0.8% lower, RR 0.99, p = 1.00, treatment 65 of 131 (49.6%), control 35 of 70 (50.0%), NNT 262, day 21.
|
|
risk of transmission, 12.1% lower, RR 0.88, p = 0.66, treatment 33 of 522 (6.3%), control 20 of 278 (7.2%), NNT 115, day 21.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Oldenburg et al., 10 Aug 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 43.0, 15 authors, study period 22 May, 2020 - 16 March, 2021, average treatment delay 7.0 days, trial NCT04332107 (history) (ACTION).
Contact: catherine.oldenburg@ucsf.edu.
Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection
JAMA, doi:10.1001/jama.2021.11517
IMPORTANCE Azithromycin has been hypothesized to have activity against SARS-CoV-2. OBJECTIVE To determine whether oral azithromycin in outpatients with SARS-CoV-2 infection leads to absence of self-reported COVID-19 symptoms at day 14. DESIGN, SETTING, AND PARTICIPANTS Randomized clinical trial of azithromycin vs matching placebo conducted from May 2020 through March 2021. Outpatients from the US were enrolled remotely via internet-based surveys and followed up for 21 days. Eligible participants had a positive SARS-CoV-2 diagnostic test result (nucleic acid amplification or antigen) within 7 days prior to enrollment, were aged 18 years or older, and were not hospitalized at the time of enrollment. Among 604 individuals screened, 297 were ineligible, 44 refused participation, and 263 were enrolled. Participants, investigators, and study staff were masked to treatment randomization. INTERVENTIONS Participants were randomized in a 2:1 fashion to a single oral 1.2-g dose of azithromycin (n = 171) or matching placebo (n = 92).
MAIN OUTCOMES AND MEASURES The primary outcome was absence of self-reported COVID-19 symptoms at day 14. There were 23 secondary clinical end points, including all-cause hospitalization at day 21. RESULTS Among 263 participants who were randomized (median age, 43 years; 174 [66%] women; 57% non-Hispanic White and 29% Latinx/Hispanic), 76% completed the trial. The trial was terminated by the data and safety monitoring committee for futility after the interim analysis. At day 14, there was no significant difference in proportion of participants who were symptom free (azithromycin: 50%; placebo: 50%; prevalence difference, 0%; 95% CI, -14% to 15%; P > .99). Of 23 prespecified secondary clinical end points, 18 showed no significant difference. By day 21, 5 participants in the azithromycin group had been hospitalized compared with 0 in the placebo group (prevalence difference, 4%; 95% CI, -1% to 9%; P = .16). CONCLUSIONS AND RELEVANCE Among outpatients with SARS-CoV-2 infection, treatment with a single dose of azithromycin compared with placebo did not result in greater likelihood of being symptom free at day 14. These findings do not support the routine use of azithromycin for outpatient SARS-CoV-2 infection.
Author Contributions: Drs Oldenburg and Arnold had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
Afshinnekoo, Bhattacharya, Burguete-García, COVID-19 drug practices risk antimicrobial resistance evolution, Lancet Microbe, doi:10.1016/S2666-5247(21)00039-2
Astale, Sata, Zerihun, Self-reported side effects following mass administration of azithromycin to eliminate trachoma in Amhara, Ethiopia: results from a region-wide population-based survey, Am J Trop Med Hyg, doi:10.1038/s41564-020-0739-4
Ayele, Gebre, House, Adverse events after mass azithromycin treatments for trachoma in Ethiopia, Am J Trop Med Hyg, doi:10.4269/ajtmh.2011.11-0056
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Cavalcanti, Zampieri, Rosa, Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2019014
Doan, Worden, Hinterwirth, Macrolide and nonmacrolide resistance with mass azithromycin distribution, N Engl J Med, doi:10.1056/NEJMoa2002606
Gyselinck, Liesenborghs, Landeloos, Direct antivirals working against the novel coronavirus: azithromycin (DAWn-AZITHRO), a randomized, multicenter, open-label, adaptive, proof-of-concept clinical trial of new antivirals working against SARS-CoV-2-azithromycin trial, Trials, doi:10.1186/s13063-021-05033-x
Johnston, Brown, Stewart, COVID-19 Early Treatment Study Team. Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: a randomized clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100773
Lachin, A review of methods for futility stopping based on conditional power, Stat Med, doi:10.1002/sim.2151
Lipsitch, Samore, Antimicrobial use and antimicrobial resistance: a population perspective, Emerg Infect Dis, doi:10.3201/eid0804.010312
Little, Agostino, Cohen, The prevention and treatment of missing data in clinical trials, N Engl J Med, doi:10.1056/NEJMsr1203730
Nehme, Braillard, Alcoba, COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings, Ann Intern Med, doi:10.7326/M20-5926
O'brien, Emerson, Hooper, Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review, Lancet Infect Dis, doi:10.1016/S1473-3099(18)30444-4
Oldenburg, Hinterwirth, Sié, Gut resistome after oral antibiotics in preschool children in Burkina Faso: a randomized, controlled trial, Clin Infect Dis, doi:10.1093/cid/ciz455
Oliver, Hinks, Azithromycin in viral infections, Rev Med Virol, doi:10.1002/rmv.2163
Recovery Collaborative, Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00149-5
Recovery Collaborative, Azithromycin in the treatment of patients admitted to the hospital with severe COVID-19: the COALITION II randomised clinical trial, Lancet, doi:10.1016/S0140-6736(21)00149-5
Sié, Dah, Bountogo, Adverse events and clinic visits following a single dose of oral azithromycin among preschool children: a randomized placebo-controlled trial, Am J Trop Med Hyg, doi:10.4269/ajtmh.20-1002
Spinato, Fabbris, Polesel, Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection, JAMA, doi:10.1001/jama.2020.6771?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2021.11517
Trial, Group, Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)00461-X
DOI record:
{
"DOI": "10.1001/jama.2021.11517",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2021.11517",
"author": [
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
},
{
"name": "Department of Epidemiology and Biostatistics, University of California, San Francisco"
}
],
"family": "Oldenburg",
"given": "Catherine E.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pathology, Stanford University School of Medicine, Stanford, California"
},
{
"name": "Clinical Virology Laboratory, Stanford Health Care, Stanford, California"
},
{
"name": "Division of Infectious Diseases and Geographic Medicine, Department of Medicine, Stanford School of Medicine, Stanford, California"
}
],
"family": "Pinsky",
"given": "Benjamin A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Brogdon",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Chen",
"given": "Cindi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Ruder",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Zhong",
"given": "Lina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Nyatigo",
"given": "Fanice",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Cook",
"given": "Catherine A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Hinterwirth",
"given": "Armin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
}
],
"family": "Lebas",
"given": "Elodie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
}
],
"family": "Redd",
"given": "Travis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
},
{
"name": "Department of Epidemiology and Biostatistics, University of California, San Francisco"
}
],
"family": "Porco",
"given": "Travis C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
},
{
"name": "Department of Epidemiology and Biostatistics, University of California, San Francisco"
}
],
"family": "Lietman",
"given": "Thomas M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
}
],
"family": "Arnold",
"given": "Benjamin F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Francis I. Proctor Foundation, University of California, San Francisco"
},
{
"name": "Department of Ophthalmology, University of California, San Francisco"
}
],
"family": "Doan",
"given": "Thuy",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
16
]
],
"date-time": "2021-07-16T15:30:44Z",
"timestamp": 1626449444000
},
"deposited": {
"date-parts": [
[
2021,
8,
10
]
],
"date-time": "2021-08-10T15:59:43Z",
"timestamp": 1628611183000
},
"indexed": {
"date-parts": [
[
2024,
9,
16
]
],
"date-time": "2024-09-16T15:54:53Z",
"timestamp": 1726502093596
},
"is-referenced-by-count": 93,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
8,
10
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2021,
8,
10
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2782166/jama_oldenburg_2021_oi_210081_1628008964.95494.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "490",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
8,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
8,
10
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1002/rmv.2163",
"article-title": "Azithromycin in viral infections.",
"author": "Oliver",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Rev Med Virol",
"key": "joi210081r1",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(18)30444-4",
"article-title": "Antimicrobial resistance following mass azithromycin distribution for trachoma: a systematic review.",
"author": "O’Brien",
"doi-asserted-by": "publisher",
"first-page": "e14",
"issue": "1",
"journal-title": "Lancet Infect Dis",
"key": "joi210081r2",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1016/S0140-6736(21)00461-X",
"article-title": "Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial.",
"author": "PRINCIPLE Trial Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1063",
"issue": "10279",
"journal-title": "Lancet",
"key": "joi210081r3",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19.",
"author": "Cavalcanti",
"doi-asserted-by": "publisher",
"first-page": "2041",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "joi210081r4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"article-title": "Azithromycin in the treatment of patients admitted to the hospital with severe COVID-19: the COALITION II randomised clinical trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "605",
"issue": "10274",
"journal-title": "Lancet",
"key": "joi210081r5",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100773",
"article-title": "Hydroxychloroquine with or without azithromycin for treatment of early SARS-CoV-2 infection among high-risk outpatient adults: a randomized clinical trial.",
"author": "Johnston",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "joi210081r6",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19.",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi210081r7",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05033-x",
"article-title": "Direct antivirals working against the novel coronavirus: azithromycin (DAWn-AZITHRO), a randomized, multicenter, open-label, adaptive, proof-of-concept clinical trial of new antivirals working against SARS-CoV-2—azithromycin trial.",
"author": "Gyselinck",
"doi-asserted-by": "publisher",
"first-page": "126",
"issue": "1",
"journal-title": "Trials",
"key": "joi210081r8",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1056/NEJMsr1203730",
"article-title": "The prevention and treatment of missing data in clinical trials.",
"author": "Little",
"doi-asserted-by": "publisher",
"first-page": "1355",
"issue": "14",
"journal-title": "N Engl J Med",
"key": "joi210081r9",
"volume": "367",
"year": "2012"
},
{
"DOI": "10.1002/(ISSN)1097-0258",
"article-title": "A review of methods for futility stopping based on conditional power.",
"author": "Lachin",
"doi-asserted-by": "publisher",
"first-page": "2747",
"issue": "18",
"journal-title": "Stat Med",
"key": "joi210081r10",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.1016/S0140-6736(21)00149-5",
"article-title": "Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "605",
"issue": "10274",
"journal-title": "Lancet",
"key": "joi210081r11",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6771",
"article-title": "Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection.",
"author": "Spinato",
"doi-asserted-by": "publisher",
"first-page": "2089",
"issue": "20",
"journal-title": "JAMA",
"key": "joi210081r12",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.7326/M20-5926",
"article-title": "COVID-19 symptoms: longitudinal evolution and persistence in outpatient settings.",
"author": "Nehme",
"doi-asserted-by": "publisher",
"first-page": "723",
"issue": "5",
"journal-title": "Ann Intern Med",
"key": "joi210081r13",
"volume": "174",
"year": "2021"
},
{
"article-title": "Adverse events and clinic visits following a single dose of oral azithromycin among preschool children: a randomized placebo-controlled trial.",
"author": "Sié",
"first-page": "1137",
"issue": "3",
"journal-title": "Am J Trop Med Hyg",
"key": "joi210081r14",
"volume": "104",
"year": "2020"
},
{
"DOI": "10.4269/ajtmh.2011.11-0056",
"article-title": "Adverse events after mass azithromycin treatments for trachoma in Ethiopia.",
"author": "Ayele",
"doi-asserted-by": "publisher",
"first-page": "291",
"issue": "2",
"journal-title": "Am J Trop Med Hyg",
"key": "joi210081r15",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.4269/ajtmh.18-0781",
"article-title": "Self-reported side effects following mass administration of azithromycin to eliminate trachoma in Amhara, Ethiopia: results from a region-wide population-based survey.",
"author": "Astale",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "3",
"journal-title": "Am J Trop Med Hyg",
"key": "joi210081r16",
"volume": "100",
"year": "2019"
},
{
"DOI": "10.1038/s41564-020-0739-4",
"article-title": "Antimicrobial resistance in the age of COVID-19.",
"doi-asserted-by": "publisher",
"first-page": "779",
"issue": "6",
"journal-title": "Nat Microbiol",
"key": "joi210081r17",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/S2666-5247(21)00039-2",
"article-title": "COVID-19 drug practices risk antimicrobial resistance evolution.",
"author": "Afshinnekoo",
"doi-asserted-by": "publisher",
"first-page": "e135",
"issue": "4",
"journal-title": "Lancet Microbe",
"key": "joi210081r18",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.3201/eid0804.010312",
"article-title": "Antimicrobial use and antimicrobial resistance: a population perspective.",
"author": "Lipsitch",
"doi-asserted-by": "publisher",
"first-page": "347",
"issue": "4",
"journal-title": "Emerg Infect Dis",
"key": "joi210081r19",
"volume": "8",
"year": "2002"
},
{
"DOI": "10.1056/NEJMoa2002606",
"article-title": "Macrolide and nonmacrolide resistance with mass azithromycin distribution.",
"author": "Doan",
"doi-asserted-by": "publisher",
"first-page": "1941",
"issue": "20",
"journal-title": "N Engl J Med",
"key": "joi210081r20",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciz455",
"article-title": "Gut resistome after oral antibiotics in preschool children in Burkina Faso: a randomized, controlled trial.",
"author": "Oldenburg",
"doi-asserted-by": "publisher",
"first-page": "525",
"issue": "3",
"journal-title": "Clin Infect Dis",
"key": "joi210081r21",
"volume": "70",
"year": "2020"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.740482290.793588147",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2782166"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of Oral Azithromycin vs Placebo on COVID-19 Symptoms in Outpatients With SARS-CoV-2 Infection",
"type": "journal-article",
"volume": "326"
}
oldenburg