Povidone-Iodine reduces COVID-19 risk: real-time meta analysis of 22 studies
, Dec 2025
PVP-I for COVID-19
14th treatment shown to reduce risk in
February 2021, now with p = 0.000000000016 from 22 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,300+ studies for
210+ treatments. c19early.org
|
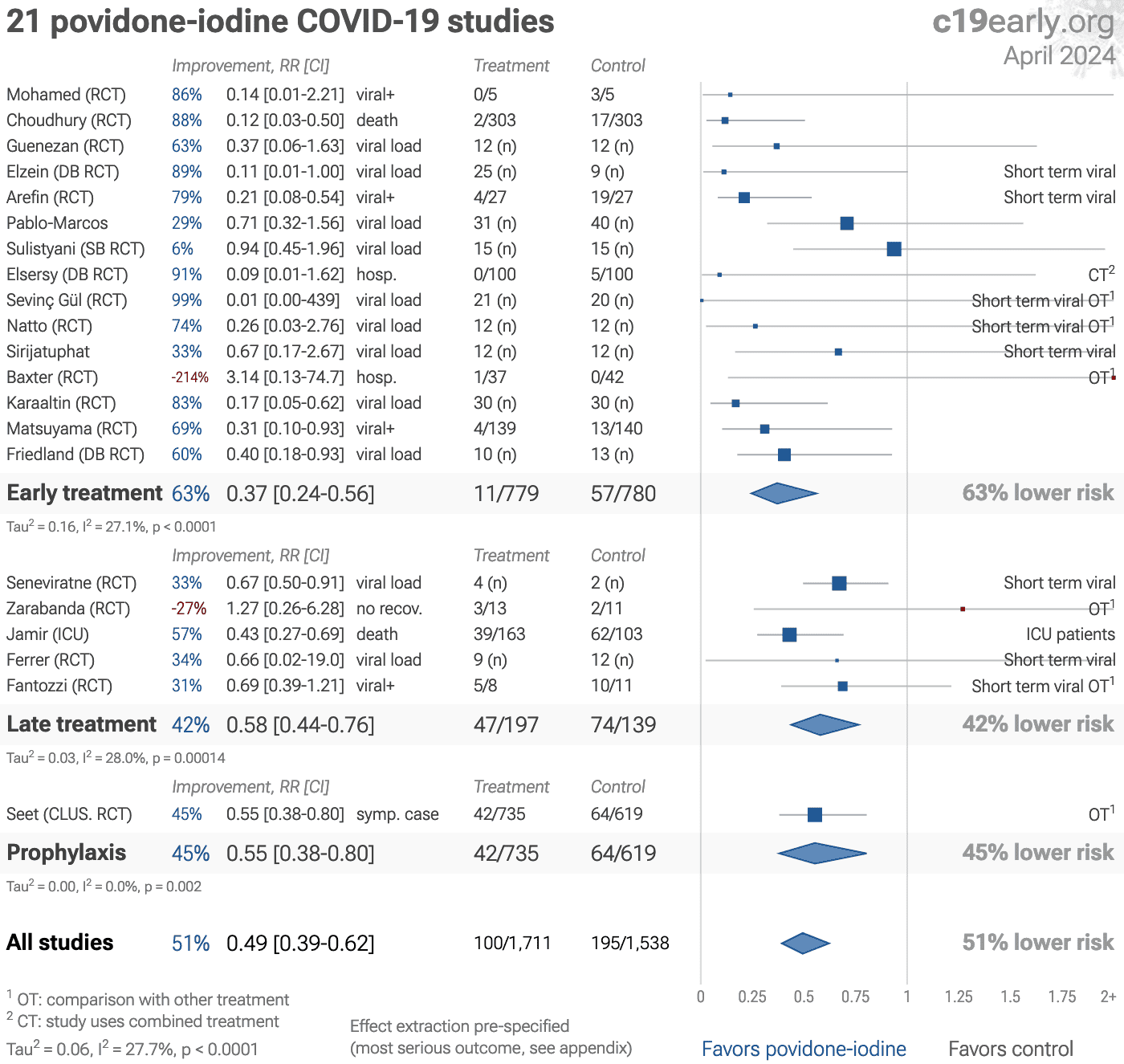
Significantly lower risk is seen for mortality, hospitalization, recovery, cases, and viral clearance. 12 studies from 12 independent teams in 10 countries show significant benefit.
Meta analysis using the most serious outcome reported shows 49% [38‑58%] lower risk. Results are similar for Randomized Controlled Trials, higher quality studies, and peer-reviewed studies. Early treatment is more effective than late treatment.
Results are very robust — in exclusion sensitivity analysis 18 of 22 studies must be excluded to avoid finding statistically significant efficacy in pooled analysis.
Control Povidone-IodinePVP-I
2 RCTs with 295 patients have not reported results (up to 3 years late)1,2.
Excessive use of PVP-I could affect thyroid function.
No treatment is 100% effective. Protocols combine safe and effective options with individual risk/benefit analysis and monitoring. Povidone-Iodine may be detrimental to the natural microbiome, raising concern for side effects, especially with prolonged or excessive use. All data and sources to reproduce this analysis are in the appendix.
Other meta analyses show significant improvements with povidone-iodine for viral load3-5 and viral clearance3.
3 meta analyses show significant improvements with povidone-iodine for viral load1-3 and
viral clearance1.
1.
Hasan et al., Effects of Chlorhexidine and Povidone-Iodine on the SARS-CoV-2 Load: A Systematic Review and Meta-analysis, European Journal of Dentistry, doi:10.1055/s-0042-1753470.
Covid Analysis et al., Dec 2025, preprint, 1 author.
