Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to Covid-19
Zachary M Holliday, Alexander P Earhart, Mohammed M Alnijoumi, Armin Krvavac, Lee-Ann H. Allen, Adam G Schrum
Frontiers in Immunology, doi:10.3389/fimmu.2021.714833
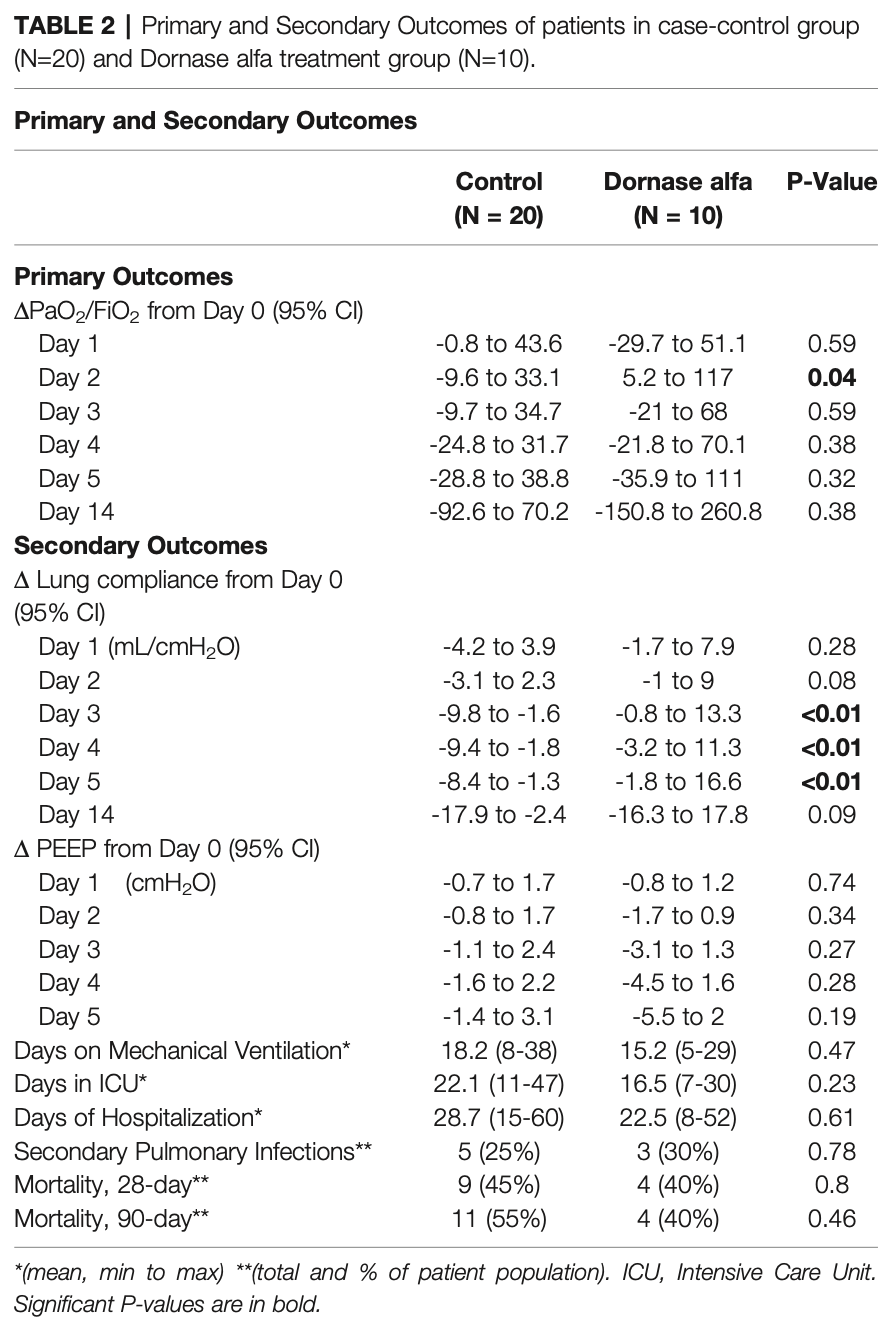
Background: The most severe cases of Coronavirus-Disease-2019 (COVID-19) develop into Acute Respiratory Distress Syndrome (ARDS). It has been proposed that oxygenation may be inhibited by extracellular deoxyribonucleic acid (DNA) in the form of neutrophil extracellular traps (NETs). Dornase alfa (Pulmozyme, Genentech) is recombinant human deoxyribonuclease I that acts as a mucolytic by cleaving and degrading extracellular DNA. We performed a pilot study to evaluate the effects of dornase alfa in patients with ARDS secondary to COVID-19. Methods: We performed a pilot, non-randomized, case-controlled clinical trial of inhaled dornase for patients who developed ARDS secondary to COVID-19 pneumonia. Results: Improvement in arterial oxygen saturation to inhaled fraction of oxygen ratio (PaO 2 /FiO 2) was noted in the treatment group compared to control at day 2 (95% CI, 2.96 to 95.66, P-value = 0.038), as well as in static lung compliance at days 3 through 5 (95% CI, 4.8 to 19.1 mL/cmH 2 O, 2.7 to 16.5 mL/cmH 2 O, and 5.3 to 19.2 mL/cmH 2 O, respectively). These effects were not sustained at 14 days. A reduction in bronchoalveolar lavage fluid (BALF) myeloperoxidase-DNA (DNA : MPO) complexes (95% CI, -14.7 to -1.32, P-value = 0.01) was observed after therapy with dornase alfa.
Conclusion: Treatment with dornase alfa was associated with improved oxygenation and decreased DNA : MPO complexes in BALF. The positive effects, however, were limited to the time of drug delivery. These data suggest that degradation of extracellular DNA associated with NETs or other structures by inhaled dornase alfa can be beneficial. We propose a more extensive clinical trial is warranted.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by University of Missouri Institutional Review Board.
AUTHOR CONTRIBUTIONS ZH, AK, AS, AE, and MA collected and analyzed the data. ZH, AE, and AS wrote the manuscript. AS, AE, AK, L-AA, and MA reviewed/edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Copyright © 2021 Holliday, Earhart, Alnijoumi, Krvavac, Allen and Schrum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
References
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps, J Exp Med,
doi:10.1084/jem.20200652Bendib, De Chaisemartin, Granger, Schlemmer, Maitre et al., Neutrophil Extracellular Traps Are Elevated in Patients With Pneumonia-Related Acute Respiratory Distress Syndrome, Anesthesiology,
doi:10.1097/ALN.0000000000002619Brinkmann, Reichard, Goosmann, Fauler, Uhlemann et al., Neutrophil Extracellular Traps Kill Bacteria, Science,
doi:10.1126/science.1092385Earhart, Holliday, Hofmann, Schrum, Consideration of Dornase Alfa for the Treatment of Severe COVID-19 Acute Respiratory Distress Syndrome, New Microbes New Infect,
doi:10.1016/j.nmni.2020.100689Ebrahimi, Giaglis, Hahn, Blum, Baumgartner et al., Markers of Neutrophil Extracellular Traps Predict Adverse Outcome in Community-Acquired Pneumonia: Secondary Analysis of a Randomised Controlled Trial, Eur Respir J,
doi:10.1183/13993003.01389-201Emanuel, Persad, Upshur, Thome, Parker et al., Fair Allocation of Scarce Medical Resources in the Time of Covid-19, N Engl J Med,
doi:10.1056/NEJMsb2005114Frantzeskaki, Armaganidis, Orfanos, Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks Between Inflammation and Coagulation, Respiration,
doi:10.1159/000453002Kambas, Chrysanthopoulou, Vassilopoulos, Apostolidou, Skendros et al., Tissue Factor Expression in Neutrophil Extracellular Traps and Neutrophil Derived Microparticles in Antineutrophil Cytoplasmic Antibody Associated Vasculitis may Promote Thromboinflammation and the Thrombophilic State Associated With the Disease, Ann Rheum Dis,
doi:10.1136/annrheumdis-2013-203430Middleton, He, Denorme, Campbell, Ng et al., Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome, Blood,
doi:10.1182/blood.2020007008Ramsey, Astley, Aitken, Colin, Dorkin, Efficacy and Safety of Short-Term Administration of Aerosolized Recombinant Human Deoxyribonuclease in Patients With Cystic Fibrosis, Am Rev Respir Dis,
doi:10.1164/ajrccm/148.1.145Schulz, Altman, Moher, Group, CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials, BMJ,
doi:10.1136/bmj.c332Shak, Capon, Hellmiss, Marsters, Baker, Recombinant Human DNase I Reduces the Viscosity of Cystic Fibrosis Sputum, Proc Natl Acad Sci,
doi:10.1073/pnas.87.23.9188Zhou, Ren, Zhang, Zhong, Xiao et al., Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients, Cell Host Microbe,
doi:10.1016/j.chom.2020.04.017DOI record:
{
"DOI": "10.3389/fimmu.2021.714833",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2021.714833",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>The most severe cases of Coronavirus-Disease-2019 (COVID-19) develop into Acute Respiratory Distress Syndrome (ARDS). It has been proposed that oxygenation may be inhibited by extracellular deoxyribonucleic acid (DNA) in the form of neutrophil extracellular traps (NETs). Dornase alfa (Pulmozyme, Genentech) is recombinant human deoxyribonuclease I that acts as a mucolytic by cleaving and degrading extracellular DNA. We performed a pilot study to evaluate the effects of dornase alfa in patients with ARDS secondary to COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We performed a pilot, non-randomized, case-controlled clinical trial of inhaled dornase for patients who developed ARDS secondary to COVID-19 pneumonia.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Improvement in arterial oxygen saturation to inhaled fraction of oxygen ratio (PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2)</jats:sub> was noted in the treatment group compared to control at day 2 (95% CI, 2.96 to 95.66, P-value = 0.038), as well as in static lung compliance at days 3 through 5 (95% CI, 4.8 to 19.1 mL/cmH<jats:sub>2</jats:sub>O, 2.7 to 16.5 mL/cmH<jats:sub>2</jats:sub>O, and 5.3 to 19.2 mL/cmH<jats:sub>2</jats:sub>O, respectively). These effects were not sustained at 14 days. A reduction in bronchoalveolar lavage fluid (BALF) myeloperoxidase-DNA (DNA : MPO) complexes (95% CI, -14.7 to -1.32, P-value = 0.01) was observed after therapy with dornase alfa.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Treatment with dornase alfa was associated with improved oxygenation and decreased DNA : MPO complexes in BALF. The positive effects, however, were limited to the time of drug delivery. These data suggest that degradation of extracellular DNA associated with NETs or other structures by inhaled dornase alfa can be beneficial. We propose a more extensive clinical trial is warranted.</jats:p></jats:sec><jats:sec><jats:title>Clinical Trial Registration</jats:title><jats:p><jats:uri>ClinicalTrials.gov</jats:uri>, Identifier: NCT04402970.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2021.714833"
],
"author": [
{
"affiliation": [],
"family": "Holliday",
"given": "Zachary M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Earhart",
"given": "Alexander P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alnijoumi",
"given": "Mohammed M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krvavac",
"given": "Armin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allen",
"given": "Lee-Ann H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schrum",
"given": "Adam G.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
10,
20
]
],
"date-time": "2021-10-20T18:58:17Z",
"timestamp": 1634756297000
},
"deposited": {
"date-parts": [
[
2021,
10,
20
]
],
"date-time": "2021-10-20T18:58:19Z",
"timestamp": 1634756299000
},
"indexed": {
"date-parts": [
[
2024,
9,
13
]
],
"date-time": "2024-09-13T05:17:13Z",
"timestamp": 1726204633974
},
"is-referenced-by-count": 42,
"issued": {
"date-parts": [
[
2021,
10,
20
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
20
]
],
"date-time": "2021-10-20T00:00:00Z",
"timestamp": 1634688000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2021.714833/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
10,
20
]
]
},
"published-online": {
"date-parts": [
[
2021,
10,
20
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1056/NEJMsb2005114",
"article-title": "Fair Allocation of Scarce Medical Resources in the Time of Covid-19",
"author": "Emanuel",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2020.100689",
"article-title": "Consideration of Dornase Alfa for the Treatment of Severe COVID-19 Acute Respiratory Distress Syndrome",
"author": "Earhart",
"doi-asserted-by": "publisher",
"first-page": "100689",
"journal-title": "New Microbes New Infect",
"key": "B2",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1126/science.1092385",
"article-title": "Neutrophil Extracellular Traps Kill Bacteria",
"author": "Brinkmann",
"doi-asserted-by": "publisher",
"journal-title": "Science",
"key": "B3",
"volume": "303",
"year": "2004"
},
{
"DOI": "10.12703/r/9-25",
"article-title": "Phagocytosis and Neutrophil Extracellular Traps",
"author": "DeLeo",
"doi-asserted-by": "publisher",
"journal-title": "Fac Rev",
"key": "B4",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1084/jem.20200652",
"article-title": "Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps",
"author": "Barnes",
"doi-asserted-by": "publisher",
"first-page": "e20200652",
"journal-title": "J Exp Med",
"key": "B5",
"volume": "217",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2016.00366",
"article-title": "Neutrophil Extracellular Traps Go Viral",
"author": "Schönrich",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "B6",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1136/annrheumdis-2013-203430",
"article-title": "Tissue Factor Expression in Neutrophil Extracellular Traps and Neutrophil Derived Microparticles in Antineutrophil Cytoplasmic Antibody Associated Vasculitis may Promote Thromboinflammation and the Thrombophilic State Associated With the Disease",
"author": "Kambas",
"doi-asserted-by": "publisher",
"journal-title": "Ann Rheum Dis",
"key": "B7",
"volume": "73",
"year": "2014"
},
{
"DOI": "10.1182/blood.2020007008",
"article-title": "Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome",
"author": "Middleton",
"doi-asserted-by": "publisher",
"journal-title": "Blood",
"key": "B8",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1073/pnas.87.23.9188",
"article-title": "Recombinant Human DNase I Reduces the Viscosity of Cystic Fibrosis Sputum",
"author": "Shak",
"doi-asserted-by": "publisher",
"journal-title": "Proc Natl Acad Sci USA",
"key": "B9",
"volume": "87",
"year": "1990"
},
{
"DOI": "10.1164/ajrccm/148.1.145",
"article-title": "Efficacy and Safety of Short-Term Administration of Aerosolized Recombinant Human Deoxyribonuclease in Patients With Cystic Fibrosis",
"author": "Ramsey",
"doi-asserted-by": "publisher",
"journal-title": "Am Rev Respir Dis",
"key": "B10",
"volume": "148",
"year": "1993"
},
{
"DOI": "10.1055/s-2004-822303",
"article-title": "Neutrophils in Innate Immunity",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "33",
"journal-title": "Semin Respir Crit Care Med",
"key": "B11",
"volume": "25",
"year": "2004"
},
{
"DOI": "10.1136/bmj.c332",
"article-title": "CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials",
"author": "Schulz",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "B12",
"volume": "340",
"year": "2010"
},
{
"DOI": "10.1016/j.chom.2020.04.017",
"article-title": "Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "883",
"journal-title": "Cell Host Microbe",
"key": "B13",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1159/000453002",
"article-title": "Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks Between Inflammation and Coagulation",
"author": "Frantzeskaki",
"doi-asserted-by": "publisher",
"journal-title": "Respiration",
"key": "B14",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1172/jci.insight.138999",
"article-title": "Neutrophil Extracellular Traps in COVID-19",
"author": "Zuo",
"doi-asserted-by": "publisher",
"first-page": "e138999",
"journal-title": "JCI Insight",
"key": "B15",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1183/13993003.01389-201",
"article-title": "Markers of Neutrophil Extracellular Traps Predict Adverse Outcome in Community-Acquired Pneumonia: Secondary Analysis of a Randomised Controlled Trial",
"author": "Ebrahimi",
"doi-asserted-by": "publisher",
"first-page": "1701389",
"journal-title": "Eur Respir J",
"key": "B16",
"volume": "51",
"year": "2018"
},
{
"DOI": "10.1097/ALN.0000000000002619",
"article-title": "Neutrophil Extracellular Traps Are Elevated in Patients With Pneumonia-Related Acute Respiratory Distress Syndrome",
"author": "Bendib",
"doi-asserted-by": "publisher",
"journal-title": "Anesthesiology",
"key": "B17",
"volume": "130",
"year": "2019"
},
{
"key": "B18",
"unstructured": "Nebulised Dornase Alfa for Treatment of COVID-19 (COVASE). ClinicalTrials.gov Identifier NCT04359654. Updated October 192020"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2021.714833/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Non-Randomized Trial of Dornase Alfa for Acute Respiratory Distress Syndrome Secondary to Covid-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "12"
}