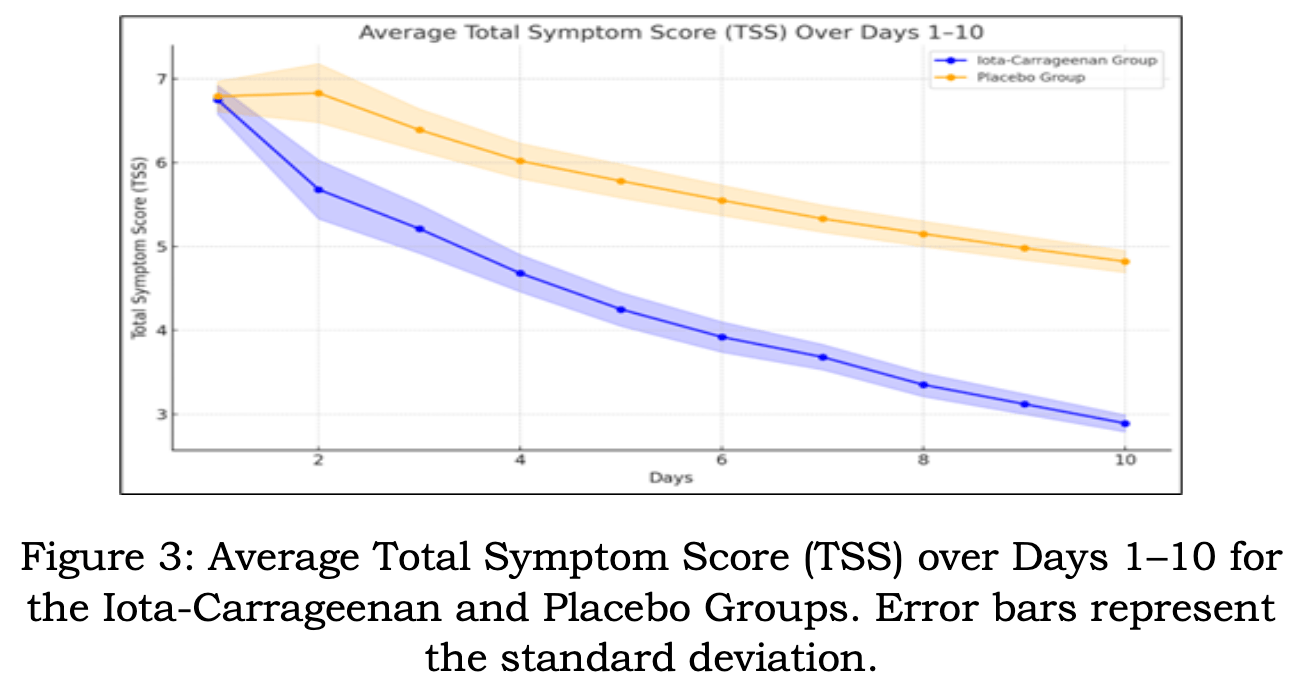
Non-COVID-19 RCT 180 patients with acute viral pharyngitis, predominantly caused by human rhinovirus (HRV), showing symptom improvement and viral load reduction with iota-carrageenan lozenges compared to placebo. Patients receiving lozenges (6 daily for up to 10 days) reported faster symptom relief based on Jackson's Total Symptom Score, particularly between days 2 and 4. Human rhinovirus (HRV)-positive participants experienced a 90.2% viral load reduction versus 72.0% in the placebo group by day 5. Adverse events were minimal and similar across groups, with mild throat irritation being most common.
Iota-carrageenan works by binding to viral particles and preventing attachment to host cells. This mechanism is not virus-specific and has shown broad-spectrum activity against other respiratory viruses, including coronaviruses and SARS-CoV-2, in preclinical studies. Lozenges provide targeted delivery to the throat, a key site of viral replication in early COVID-19. This localized action could help reduce viral load in the upper respiratory tract, potentially impacting transmission and disease progression.
Karna et al., 30 Dec 2024, Double Blind Randomized Controlled Trial, placebo-controlled, peer-reviewed, 2 authors.